
Диметиловый эфир диэтиленгликоля
- английское имяDiglyme
- CAS №111-96-6
- CBNumberCB4302276
- ФормулаC6H14O3
- мольный вес134.17
- EINECS203-924-4
- номер MDLMFCD00008503
- файл Mol111-96-6.mol
| Температура плавления | -64 °C (lit.) |
| Температура кипения | 162 °C (lit.) |
| плотность | 0.944 g/mL at 20 °C (lit.) 0.939 g/mL at 25 °C (lit.) |
| плотность пара | 4.6 (vs air) |
| давление пара | 3 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 134.6 °F |
| температура хранения | Store below +30°C. |
| растворимость | Chloroform (Sparingly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| форма | Liquid |
| цвет | ≤10(APHA) |
| РН | 7 (20°C) |
| Относительная полярность | 0.244 |
| Запах | Mild ethereal. |
| Пределы взрываемости | 1.4-17.4%(V) |
| Скорость испарения | 0.36 |
| Вязкость | 1.14 mPa s 20 C |
| Растворимость в воде | Miscible |
| Чувствительный | Hygroscopic |
| λмакс | λ: 225 nm Amax: 1.00 λ: 240 nm Amax: 0.50 λ: 260 nm Amax: 0.20 λ: 280 nm Amax: 0.08 λ: 320-400 nm Amax: 0.01 |
| Мерк | 14,3165 |
| БРН | 1736101 |
| Диэлектрическая постоянная | 7.2999999999999998 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. May be air or light sensitive. |
| ИнЧИКей | SBZXBUIDTXKZTM-UHFFFAOYSA-N |
| LogP | -0.36 at 25℃ |
| Surface tension | 24.56mN/m at 298.15K |
| Справочник по базе данных CAS | 111-96-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2-5 |
| FDA UNII | M4BH3X0MVZ |
| Справочник по химии NIST | Ethane, 1,1'-oxybis[2-methoxy-(111-96-6) |
| Система регистрации веществ EPA | Diethylene glycol dimethyl ether (111-96-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T | |||||||||
| Заявления о рисках | 60-61-10-19 | |||||||||
| Заявления о безопасности | 53-45 | |||||||||
| РИДАДР | UN 3271 3/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | KN3339000 | |||||||||
| F | 3-10-23 | |||||||||
| Температура самовоспламенения | 370 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29091990 | |||||||||
| Банк данных об опасных веществах | 111-96-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 4760 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Диметиловый эфир диэтиленгликоля химические свойства, назначение, производство
Описание
Bis (2-methoxyethyl) ether, also known as diglyme, is a linear aliphatic diether widely used as a solvent and present as a clear liquid at room temperature with a mild ether odor. The compound is notknown to occur in nature. It is synthesized from ethylene oxide and methanol in the presence of either acidic or basic catalysts. The reaction is based on the classic Williamson ether synthesis. It can also be produced from diethylene glycol and dimethyl sulfate. In June 2012, ECHA proposed addition of diglyme to the REACH very high concern list.Химические свойства
Diethylene glycol dimethyl ether is a clear, water-white neutral liquid of faint, pleasant odor. This ether may be used as a solvent for alkali metal hydrides for use in such reactions as reduction, alkylation and condensation. It may also be used as a lacquer solvent.Использование
Diethylene glycol dimethyl ether is used as a solvent in organic reactions due to its stability towards higher pH and its high boiling point. It is particularly involved in reactions utilizing organometallic reagents such as Grignard reactions and metal hydride reductions. It is also a solvent for hydroboration reactions with diborane.Определение
ChEBI: A polyether that is the dimethyl ether derivative of diethylene glycol.Общее описание
Colorless watery liquid with a pleasant odor. Floats and mixes with water.Реакции воздуха и воды
Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. A mixture of liquid air and diethyl ether exploded spontaneously, [MCA Case History 616(1960)]. Water soluble.Профиль реактивности
A violent explosion occurred when lithium aluminum hydride was being used to dry 2-Methoxyethyl ether. The ignition may have occurred due to the presence of large amounts of water or perhaps peroxide formed in the ether. About 75% of the ether had been removed when the explosion occurred, [MCA Case History 1494 (1968)].Угроза здоровью
INGESTION (severe cases): nausea, vomiting, abdominal cramps, weakness progressing to coma.Пожароопасность
2-Methoxyethyl ether is combustible.Химическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Синтез
265 g (2.5 mol) of diethylene glycol, 320 g (10.0 mol) of methanol, and 14.3 g (0.0125 equivalents) of NAFION 1100 EW Polymer (H+ form) were charged to a one-liter autoclave. After sealing and pressure testing, the contents of the autoclave were agitated, and the autoclave was pressurized to 100 psi with nitrogen. After 5 minutes of agitation, the autoclave was depressurized. This process was repeated two more times to ensure complete deoxygenation. After deoxygenation, the autoclave was heated to a temperature of 198 ℃, and the contents of the autoclave were agitated at 1900 rpm for 5 hours at temperature (198-200 ℃). A pressure of 810 psi was obtained. After 5 hours, the autoclave was cooled and sampled. By analysis, a total of 77.2% by weight of the diethylene glycol (1.93 moles) was converted in the reaction, producing 0.335 mol of Diglyme. The co-product includes 1,4 dioxane and the intermediate diethylene glycol monomethyl ether.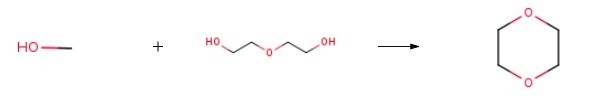
Экологическая судьба
The metabolite 2-methoxyacetic acid, which is generated from 2-methroxyethanol by the reaction of alcohol dehydrogenase, may be important for the toxic effects. It can undergo activation to methoxyacetyl coenzyme A and enter the Krebs cycle or fatty acid biosynthesis. Several metabolites of 2-methoxyethanol, such as 2-methoxy-N-acetyl glycine, have been identified that support this pathway. Thus, 2-methoxyacetic acid may interfere with essential metabolic pathways of the cell, and it was hypothesized that this causes the testicular lesions and malformations in experimental animals.Методы очистки
Dry diglyme with NaOH pellets or CaH2, then reflux with, and distil (under reduced pressure) it from Na, CaH2, LiAlH4, NaBH4 or NaH. These operations are carried out under N2. The amine-like odour of diglyme has been removed by shaking with a weakly acidic ion-exchange resin (Amberlite IR-120) before drying and distilling. Addition of 0.01% NaBH4 to the distillate inhibits peroxidation. Purify it also as for dioxane. It has been passed through a 12-in column of molecular sieves to remove water and peroxides. [Beilstein 1 IV 2393.]Диметиловый эфир диэтиленгликоля запасные части и сырье
сырьё
1of2
запасной предмет
- 5-АМИНОМЕТИЛ-ПИРРОЛИДИН-2-ОН
- 2-бром-6-(1H-пиразол-1-ил)пиридин
- 1- (2-метоксифенил) пиперазина гидробромид
- (2,5-ДИМЕТИЛ-1,3-ОКСАЗОЛ-4-YL) МЕТАНОЛ
- 6,6'-Dimethyl-2,2'-dipyridyl
1of3
Диметиловый эфир диэтиленгликоля поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0519-85551759 +8613506123987 |
China | 8829 | 58 | ||
| +86-5638152626 +86-17756305689 |
China | 10 | 58 | ||
| +8617732866630 | China | 8773 | 58 | ||
| +8613288715578 | China | 12554 | 58 | ||
| +86-13131129325 | China | 5251 | 58 | ||
| +86-17331933971 | China | 2472 | 58 | ||
| +86-15350851019; +8615350851019 |
China | 1000 | 58 | ||
| +86-13322801610 +86-15521457061 |
China | 248 | 58 | ||
| +8613336195806 | China | 29733 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21622 | 55 |
Диметиловый эфир диэтиленгликоля Обзор)
- МОНОБУТИЛОВЫЙ ЭФИР ДИЭТИЛЕНГЛИКОЛЯ
- 2-(2-хлорэтокси)этанол
- Диэтиленгликоля диакрила
- ЭТАН
- Диэтиленгликоля
- р-Анизидин
- P-анисовый альдегид
- Монометиловый эфир диэтиленгликоля
- (Трифторметокси)бензол
- 1,2-диметоксиэтан
1of4