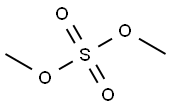
Диметил сульфа
- английское имяDimethyl sulfate
- CAS №77-78-1
- CBNumberCB9854316
- ФормулаC2H6O4S
- мольный вес126.13
- EINECS201-058-1
- номер MDLMFCD00008416
- файл Mol77-78-1.mol
| Температура плавления | -32 °C |
| Температура кипения | 188 °C(lit.) |
| плотность | 1.333 g/mL at 25 °C(lit.) |
| плотность пара | 4.3 (vs air) |
| давление пара | 0.7 mm Hg ( 25 °C) |
| показатель преломления | n |
| Fp | 182 °F |
| температура хранения | 2-8°C |
| растворимость | ethanol: 0.26 g/mL, clear, colorless |
| форма | Liquid |
| цвет | Clear colorless |
| Запах | Almost odorless |
| Пределы взрываемости | 3.6-23.2% (v/v) |
| Растворимость в воде | 2.8 g/100 mL (18 ºC) |
| Мерк | 13,3282 |
| БРН | 635994 |
| Пределы воздействия | TLV/PEL-TWA skin 0.1 ppm (0.52 mg/m3 ) (ACGIH, OSHA, NIOSH) IDLH 10 ppm (NIOSH). |
| Диэлектрическая постоянная | 55.0(20℃) |
| Стабильность | Stable; combustible. Incompatible with strong oxidizing agents, strong bases including ammonia. Moisture-sensitive. |
| Справочник по базе данных CAS | 77-78-1(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | DIMETHYL SULFATE |
| Рейтинг продуктов питания EWG | 5-9 |
| FDA UNII | JW5CW40Z50 |
| Предложение 65 Список | Dimethyl Sulfate |
| МАИР | 2A (Vol. 4, Sup 7, 71) 1999 |
| Справочник по химии NIST | Sulfuric acid, dimethyl ester(77-78-1) |
| Система регистрации веществ EPA | Dimethyl sulfate (77-78-1) |
| UNSPSC Code | 12352100 |
| NACRES | NA.21 |
| Коды опасности | T+ | |||||||||
| Заявления о рисках | 45-25-26-34-43-68 | |||||||||
| Заявления о безопасности | 53-45-61 | |||||||||
| РИДАДР | UN 1595 6.1/PG 1 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.1 ppm (0.5 mg/m3) [skin] | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | WS8225000 | |||||||||
| F | 21 | |||||||||
| Температура самовоспламенения | 495 °C | |||||||||
| Класс опасности | 6.1(a) | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 29209090 | |||||||||
| Банк данных об опасных веществах | 77-78-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 440 mg/kg (Smyth) | |||||||||
| ИДЛА | 7 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H350:Может вызывать раковые заболевания.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H330:Смертельно при вдыхании.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Диметил сульфа химические свойства, назначение, производство
Описание
Dimethyl sulfate is a colorless, oily liquid with a faint, onionlike odor. It is soluble in water, ether, dioxane, acetone, benzene, and other aromatic hydrocarbons, miscible with ethanol, and sparingly soluble in carbon disulfide. It is stable under normal temperatures and pressures, but hydrolyzes rapidly in water at or above 18 ℃.Dimethyl sulfate has been produced commercially since at least the 1920s. One production method is continuous reaction of dimethyl ether with sulfur trioxide. In 2009, dimethyl sulfate was produced by 33 manufacturers worldwide, including 1 in the United States, 14 in China, 5 in India, 5 in Europe, 6 in East Asia, and 2 in Mexico, and was available from 44 suppliers, including 16 US suppliers. There are no data on US imports or exports of dimethyl sulfate. Reports filed from 1986 through 2002 under the US Environmental Protection Agency’s Toxic Substances Control Act Inventory Update Rule indicate that US production plus imports of dimethyl sulfate totaled 10–50 million pounds. The simplest way of synthesizing dimethyl sulfate is by esterification of sulfuric acid with methanol as follows:2CH3OH+ H2SO4→(CH3)2SO4 + 2H2O
Химические свойства
Dimethyl sulfate is essentially odorless. The specific gravity of this colorless, corrosive, oily liquid is 1.3322 g/cm3. Dimethyl sulfate is soluble in ether, dioxane, acetone, benzene, and other aromatic hydrocarbons. It is sparingly soluble in carbon disulfide and aliphatic hydrocarbons, and only slightly soluble in water (28 g/l at 18 °C) (O'Neil, 2006).Использование
Dimethyl sulfate is a strong alkylating agent and might also react with the carboxylic acid substrate, further reducing the DMS concentration in the mixture. It is used as a methylating agent in themanufacture of many organic compounds,such as, phenols and thiols. Also, it is used inthe manufacture of dyes and perfumes, andas an intermediate for quaternary ammoniumsalts. It was used in the past as a militarypoison.прикладной
Dimethyl Sulfate is a diester of methanol and sulfuric acid. Dimethyl Sulfate is commonly used as a reagent for the methylation of phenols, amines, and thiols. Dimethyl Sulfate is an effective and widely used probe for sequence-specific protein-DNA interactions.Определение
ChEBI: Dimethyl sulfate is the dimethyl ester of sulfuric acid. It has a role as an alkylating agent and an immunosuppressive agent.Подготовка
Dimethyl sulfate is prepared by distillation of an oleum/methanol mixture; technical production using dimethyl ether and SO3 has also been reported (NLM, 2013).Общее описание
Dimethyl sulfate is a colorless oily liquid, odorless to a faint onion-like odor. Dimethyl sulfate is very toxic by inhalation. Dimethyl sulfate is a combustible liquid and has a flash point of 182°F. Dimethyl sulfate is slightly soluble in water and decomposed by water to give sulfuric acid with evolution of heat. Dimethyl sulfate is corrosive to metals and tissue.Реакции воздуха и воды
Water soluble.Профиль реактивности
Pure Dimethyl sulfate and concentrated aqueous ammonia react extremely violently with one another, as is the case for tertiary organic bases, [NFPA 491M, 1991]. Dimethyl sulfate ignites in contact with unheated barium chlorite, due to the rapid formation of unstable methyl chlorite. The product of methylating an unnamed material at 110°C was alloyed to remain in a reactor for 80 min. before the reactor exploded. This involved a sulfur ester such as Dimethyl sulfate, [MCA Case History No. 1786].Угроза здоровью
Dimethyl sulfate is extremely hazardous because of its lack of warning properties and delayed toxic effects. The vapor of this compound is extremely irritating to the skin, eyes, and respiratory tract, and contact with the liquid can cause very severe burns to the eyes and skin. Ingestion of dimethyl sulfate causes burns to the mouth, throat, and gastrointestinal tract. The effects of overexposure to dimethyl sulfate vapor may be delayed. After a latent period of 10 hours or more, headache and severe pain to the eyes upon exposure to light may occur, followed by cough, tightness of the chest, shortness of breath, difficulty in swallowing and speaking, vomiting, diarrhea, and painful urination. Fatal pulmonary edema may develop. Systemic effects of dimethyl sulfate include damage to the liver and kidneys. Dimethyl sulfate is listed by IARC in Group 2A ("probable human carcinogen") and is classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard. Data indicate that dimethyl sulfate does not specifically harm unborn animals; dimethyl sulfate is not a developmental toxin. It is a strong alkylating agent and does produce genetic damage in animals and in bacterial and mammalian cell cultures.Воспламеняемость и взрывоопасность
Dimethyl sulfate is a combustible liquid (NFPA rating = 2). Toxic dimethyl sulfate vapors are produced in a fire. Carbon dioxide or dry chemical extinguishers should be used to fight dimethyl sulfate fires.Канцерогенность
Dimethyl sulfate is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.Экологическая судьба
Chemical/Physical. Hydrolyzes in water (half-life = 1.2 h) to methanol and sulfuric acid (Robertson and Sugamori, 1966) via the intermediate methyl sulfuric acid (Du Pont, 1999a)хранилище
work with dimethyl sulfate should be conducted in a fume hood to prevent exposure by inhalation, and appropriate impermeable gloves and safety goggles should be worn at all times to prevent skin and eye contact.Несовместимости
Dimethyl sulfate can react violently with ammonium hydroxide, sodium azide, and strong oxidizers.Утилизация отходов
Excess dimethyl sulfate and waste material containing this substance should be placed in a covered metal container, clearly labeled, and handled according to your institution's waste disposal guidelines.Диметил сульфа запасные части и сырье
сырьё
1of2
запасной предмет
- Cationic Brilliant Blue RL
- 2,4,5-триметоксинитробензол
- 3'-DIMETHYLAMINOACETOPHENONE
- 3,5-DIBROMO-2-METHOXYBENZALDEHYDE
- 4-Хлор-2 ,5-диметоксианилина
- 3,4,5-Trimethoxybenzhydrazide
- 3-диметиламинобензойной кислоты
- Фурсультиамин
- Амидопирин
- BENZOIC 2-BENZOYL-1,2-DIMETHYLHYDRAZIDE
- 2-(4-(ACETYLAMINO)PHENYL)PROPIONITRILE
- 6-AMINO-2-METHYLTHIO-3-METHYLURACIL
- O-анисовая кислота
- 3,5-диметоксибензойная кислота
- 2- (4-нитрофенил) пропиононитрил
- 5-(Dimethylamino)-1-naphthalenesulfonic acid
- SCOPARONE
- 2-(4-аминофенил)пропиононитрил
- antistatic Agent TM
- Cationic surface active agent
- trimethyl lauroylaminopropyl ammonium methylsulfate
- 2,4-диметоксибензойной кислоты
- BIFENAZATE
- 4-METHYLDIBENZOTHIOPHENE
- α-диметоксиметилметоксипропионитрил
- Метиловый эфир 4-ацетамидо-5-хлор-2-метоксибензойной кислоты
- Methyl 4-acetamido-2-methoxybenzoate
- 1-ХЛОРМЕТИЛ-2,3,4-ТРИМЕТОКСИБЕНЗОЛ
- МЕТИЛ ЭФИР 3,4-ДИГИДРОКС-5-МЕТОКСИБЕНЗОЙНОЙ КИСЛОТЫ
- 5-Хлор-2-methylaminobenzophenone
1of8
Диметил сульфа поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-15662691337 +86-15662695772 |
China | 998 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 |
Диметил сульфа Обзор)
1of4