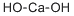
альция гидроксид
- английское имяCalcium hydroxide
- CAS №1305-62-0
- CBNumberCB9853016
- ФормулаCaH2O2
- мольный вес74.09
- EINECS215-137-3
- номер MDLMFCD00010901
- файл Mol1305-62-0.mol
| Температура плавления | 580 °C |
| Температура кипения | 2850 °C |
| плотность | 2.24 g/mL at 25 °C (lit.) |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | 1.7g/l |
| форма | Solid |
| пка | 12.6[at 20 ℃] |
| цвет | White |
| Запах | Odorless |
| Водородный показатель | 12.4 |
| РН | 11.27(1 mM solution);12.2(10 mM solution);12.46(100 mM solution); |
| Растворимость в воде | 1.65 g/L (20 ºC) |
| Чувствительный | Air Sensitive |
| Мерк | 14,1673 |
| Константа произведения растворимости (Ksp) | pKsp: 5.26 |
| Пределы воздействия | ACGIH: TWA 5 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 5 mg/m3 |
| Стабильность | Stable. Incompatible with strong acids. |
| Справочник по базе данных CAS | 1305-62-0(CAS DataBase Reference) |
| FDA 21 CFR | 184.1205; 582.1205; 310.545 |
| Вещества, добавляемые в пищу (ранее EAFUS) | CALCIUM HYDROXIDE |
| Специальный комитет по веществам GRAS | Calcium hydroxide |
| Рейтинг продуктов питания EWG | 1-4 |
| FDA UNII | PF5DZW74VN |
| Справочник по химии NIST | Calcium dihydroxide(1305-62-0) |
| Система регистрации веществ EPA | Calcium hydroxide (1305-62-0) |
| Коды опасности | Xi,C | |||||||||
| Заявления о рисках | 41-34-37/38 | |||||||||
| Заявления о безопасности | 26-39-45-36/37/39-27 | |||||||||
| РИДАДР | 3262 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | EW2800000 | |||||||||
| F | 34 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2825 90 19 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 1305-62-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 7.34 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H318:При попадании в глаза вызывает необратимые последствия.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
альция гидроксид химические свойства, назначение, производство
Описание
Calcium Hydroxide is a colorless crystal or white powder, obtained when CaO (called lime or quicklime) is mixed, or “slaked” with water. It can also be precipitated by mixing CaCl2 andNaOH.The nameof the natural,mineral formis “portlandite”. It is a relatively rare mineral, known from some volcanic, plutonic, and metamorphic rocks. It has also been known to be formed in burning coal dumps.Химические свойства
Calcium hydroxide occurs as a white or almost white, crystalline or granular powder. It has a bitter, alkaline taste. Calcium hydroxide readily absorbs carbon dioxide to form calcium carbonate.Физические свойства
Soft white crystalline powder; hexagonal; density 2.34 g/cm3; slightly bitter taste; loses water when heated at elevated temperatures (580°C); slightly soluble in water; Ksp 1.2x10-14; aqueous solution alkaline; soluble in glycerol and acids; insoluble in alcohol.Использование
calcium hydroxide is an inorganic base used to adjust the pH of a product, it can also serve as a topical astringent and alkali in solutions or lotions. It can burn the skin and eyes.Определение
Lime water: A solution of calcium hydroxide in water. If carbon dioxide is bubbled through lime water a milky precipitate of calcium carbonate forms. Prolonged bubbling of carbon dioxide turns the solution clear again as a result of the formation of soluble calcium hydrogencarbonate (Ca(HCO3)2).Методы производства
Calcium hydroxide is manufactured by adding water to calcium oxide, a process called slaking.Общее описание
Odorless white granules. Sinks in water.Реакции воздуха и воды
Water soluble. The amount of heat generated by hydrolysis may be large.Профиль реактивности
The nitroparaffins, nitromethane, nitropropane, etc. form salts with inorganic bases such as Calcium hydroxide . The dry salts are explosive [Chem. Eng. news 30:2344 1952]. Bases are chemically similar to sodium hydroxide (NaOH) or sodium oxide (Na2O). They neutralize acids exothermically to form salts plus water. When soluble in water they give solutions having a pH greater than 7.0. Mixing these materials with water can generate troublesome amounts of heat as the base is dissolved or diluted. Bases react with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. Bases may initiate polymerization reactions in polymerizable organic compounds, especially epoxides). They may generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. Materials of this group often serve as catalysts. A strong base. Forms caustic solution in water [Merck 11th ed. 1989].Опасность
Skin, eye, and upper respiratory tract irri- tant, avoid inhalation.Угроза здоровью
Dust irritates eyes, nose and throat.Сельскохозяйственное использование
Calcium hydroxide, [Ca(OH)2], also called slaked lime or hydrated lime, is a white solid that dissolves sparingly in water. It is manufactured by adding water to calcium oxide, a process that emits heat and is known as slaking.Calcium hydroxide is a cheap alkali, used for neutralizing the acidity of acid soils and in the manufacture of mortar, white wash, bleaching powder and glass. It is an excellent absorbent for carbon dioxide to produce insoluble calcium carbonate and has a neutralizing value of 179%, compared to 100% of pure calcium carbonate.
Фармацевтические приложения
Calcium hydroxide is a strong alkali and is used as a pharmaceutical pH adjuster/buffer and antacid in topical medicinal ointments, creams, lotions, and suspensions, often as an aqueous solution (lime water). It forms calcium soaps of fatty acids, which produce water-in-oil emulsions (calamine liniment), and it is also used as a topical astringent.Calcium hydroxide is a common cosmetic ingredient in hairstraightening and hair-removal products, and in shaving preparations.
1) In dentistry, it is used as a filling agent and in dental pastes to encourage deposition of secondary dentine. Calcium hydroxide was traditionally used as an escharotic in Vienna Paste.
Профиль безопасности
Mildly toxic by ingestion. A severe eye irritant. A skin, mucous membrane, and respiratory system irritant. Mutation data reported. Causes dermatitis. Dust is considered to be a significant industrial hazard. A common air contaminant. Violent reaction with maleic anhydride, nitroethane, nitromethane, nitroparaffins, nitropropane, phosphorus. Reaction with polychloiinated phenols + potassium nitrate forms extremely toxic products. See also CALCIUM COMPOUNDSБезопасность
Calcium hydroxide is used in oral and topical pharmaceutical formulations. It is mildly toxic by ingestion. In the pure state, calcium hydroxide is a severe skin, eye, and respiratory irritant, and it is corrosive, causing burns. Typical exposure limits are TVL 5 mg/m3 in air.LD50 (mouse, oral): 7.3 g/kg
LD50 (rat, oral): 7.34 g/kg
Возможный контакт
Calcium hydroxide is used in agriculture and in fertilizer manufacture; it is used in the formulation of mortar, plasters, and cements; it is used as a scrubbing and neutralizing agent in the chemical industry. For making insecticides, acaricides, and products to control arthropodsхранилище
Calcium hydroxide should be stored in an airtight container, in a cool, dry, well-ventilated place. Calcium hydroxide powder may be sterilized by heating for 1 hour at a temperature of at least 1608℃.Перевозки
UN3262 Corrosive solid, basic, inorganic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name requiredМетоды очистки
Heat analytical grade calcium carbonate at 1000o during 1hour. Allow the resulting oxide to cool and add slowly to water. Heat the suspension to boiling, cool and filter through a sintered glass funnel of medium porosity (to remove soluble alkaline impurities). Dry the solid at 110o and crush it to a uniformly fine powder. [Ehrlich in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 934 1963.]Несовместимости
Incompatible with strong acids, maleic anhydride, phosphorus, nitroethane, nitromethane, nitroparaffins, and nitropropane. Calcium hydroxide can be corrosive to some metals.Утилизация отходов
Landfill or admixture with acid industrial wastes prior to lagooningРегуляторный статус
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (intravenous and subcutaneous injections; oral suspensions and tablets; topical emulsions and creams). Included in parenteral preparations licensed in the UK.альция гидроксид запасные части и сырье
сырьё
запасной предмет
- Calcium-based grease
- CALCIUM5INOSINATE
- Гидрохлорид 4-гидроксибензамидина
- CALCIUM IODIDE HYDRATE
- CALCIUM BROMATE
- MOLECULAR SIEVES
- Vicnna lime
- 1,2:3,4-ди-O-изопропилиден-D-галактопираноза
- Кальций азотнокислый
- Кальция глицерофосфат
1of8
альция гидроксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-371-86557731 +86-13613820652 |
China | 20314 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 970 | 58 | |
| +8615530197691 | China | 105 | 58 | |
| +86-518-85110578 +8618805133257 |
China | 132 | 60 | |
| +86-0551-65418679 +86-18949832763 |
China | 2989 | 55 | |
| 18017610038 | CHINA | 3620 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29897 | 58 | |
| 18871490254 | CHINA | 28180 | 58 | |
| +86-19930503282 | China | 8826 | 58 | |
| +86-592-6051114 +8618959220845 |
China | 6387 | 58 |