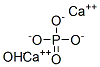
Hydroxylapatite
- английское имяHydroxyapatite
- CAS №1306-06-5
- CBNumberCB2131094
- ФормулаCa5HO13P3
- мольный вес502.31
- EINECS215-145-7
- номер MDLMFCD00010904
- файл Mol1306-06-5.mol
| Температура плавления | 1100 °C(lit.) |
| плотность | 3.076 g/cm3(Temp: 18 °C) |
| температура хранения | 2-8°C |
| растворимость | H2O: 0.3 mg/mL, clear, colorless |
| форма | solid |
| цвет | White |
| Растворимость в воде | insoluble H2O [MER06] |
| Мерк | 13,3500 |
| InChI | InChI=1S/2Ca.H3O4P.H2O/c;;1-5(2,3)4;/h;;(H3,1,2,3,4);1H2/q2*+2;;/p-4 |
| ИнЧИКей | CGMRCMMOCQYHAD-UHFFFAOYSA-J |
| SMILES | [Ca+2].[Ca+2].P([O-])([O-])([O-])=O.[OH-] |
| Справочник по базе данных CAS | 1306-06-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 91D9GV0Z28 |
| Система регистрации веществ EPA | Hydroxylapatite (Ca5(OH)(PO4)3) (1306-06-5) |
| UNSPSC Code | 41116133 |
| NACRES | NA.77 |
| Коды опасности | Xi |
| Заявления о рисках | 36/37/38 |
| Заявления о безопасности | 26-36 |
| WGK Германия | 3 |
| RTECS | MY8434000 |
| F | 3-10 |
| TSCA | Yes |
| кода HS | 28352600 |
| Банк данных об опасных веществах | 1306-06-5(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P405:Хранить в недоступном для посторонних месте.
Hydroxylapatite химические свойства, назначение, производство
Описание
Calcium hydroxyapatite has a unique structure in that it is conductive along the hydroxide channels. OH- ions lie at (1/4/,1/4,1/4) and (3/4/,3/4,3/4) on the c-axis and charge-carrying protons are responsible for the observed conductivities in M10(PO4)6(OH)2. The H+ migration between the electroattractive ion (O2) to give molecular H2O in matrix channels carries charge and the resulting conductivity.The unit cell consists of two triangular prismatic subcells forming a rhombic prism with vertical sides. There are two horizontal mirror planes at the OH levels of 1/4 and 3/4 of the c-axis. In addition, there is a center of inversion exactly in the center of each vertical face of each subcell.
Химические свойства
white powderВхождение
Occurs in nature as the minerals: oxydapatit, voelicherite, whitlockite.Использование
In the 1970s, it was found that sintered calcium hydroxyapatite, (Ca10(PO4)6(OH)2 (abbreviated as CaHap), possessed excellent biocompatibility and nontoxicity with femur and mandible bones. Since then, CaHap has been used as a biomaterial for artificial teeth and bones and as a filler for cements and polymers. Today, bone fillers made of CaHap are widely used in the medical and dental fields. In the 1980s, it was found that sintered CaHap has a good compatibility with skin tissues. Hence, percutaneous devices based on CaHap have been developed and applications have included continuous ambulatory peritoneal dialysis and intravenous hyperalimentation systems, blood pressure measurement and blood access for nutrition. Recently, many researchers have studied the chemistry of apatites, particularly CaHap, and have found various applications, such as artificial teeth and bones, ion exchangers, adsorbents for chromatography to separate proteins and enzymes, catalysts, ionic conductors, temperature and gas sensors, etc.Определение
The major constituent of bone and tooth mineral. It is finely divided, crystalline, nonstoichiometric material rich in surface ions (carbonate, magnesium, citrate), which are readily replaced by fluoride ion, thus affording protection to the teeth.Подготовка
The technical product is also known as “bone ash.” Commercial preparation from phosphate rock.Общее описание
Bone and tooth implant materials have been prepared from polycrystalline hydroxyapatite. The compressive, flexural, torsional and dynamic torsional strengths of polycrystalline hydroxyapatite were investigated. Electrophoretic deposition of hydroxyapatite on to flat titanium plate material has been studied.Фармацевтические приложения
Modified hydroxylapatite, also frequently called hydroxyapatite and better known as bone mineral, makes up ~50% of our bones. Hydroxylapatite is a natural form of the mineral calcium apatite, whose formula is usually denoted as Ca10(PO4)6(OH)2. Modifications of hydroxylapatite can also be found in the teeth, and a chemically identical substance is often used as filler for replacement of bones, and so on. Nevertheless, despite similar or identical chemical compositions, the response of the body to these compounds can be quite different.Hydroxylapatite запасные части и сырье
запасной предмет
Hydroxylapatite поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8615309206328 | China | 298 | 58 | ||
| +undefined18013301590 | China | 2448 | 58 | ||
| +8617732866630 | China | 8773 | 58 | ||
| +86-29-81148696 +86-17392712697 |
China | 3889 | 58 | ||
| +8618531123677 | China | 5853 | 58 | ||
| +86-89586680 +86-13289823923 |
China | 8982 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1001 | 58 | ||
| +86-0311-87836622 +86-18712993135 |
China | 8051 | 58 | ||
| +8615531151365 | China | 18126 | 58 | ||
| +undefined18602966907 | China | 997 | 58 |