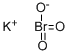
Бромат калия
- английское имяPotassium bromate
- CAS №7758-01-2
- CBNumberCB5281569
- ФормулаBrKO3
- мольный вес167
- EINECS231-829-8
- номер MDLMFCD00011359
- файл Mol7758-01-2.mol
| Температура плавления | 350 °C |
| плотность | 3.27 |
| Плотность накопления | 1400kg/m3 |
| температура хранения | Store below +30°C. |
| растворимость | Potassium bromate is highly soluble in water (7.5 g/100 mL at 25°C; 49.8 g/100 mL at 100°C), slightly soluble in ethanol, and almost insoluble in acetone; it is very stable when dissolved in water at room temperature. |
| форма | Powder/Solid |
| Удельный вес | 3.27 |
| цвет | White crystals or granules |
| РН | 5.0-9.0 (25℃, 50mg/mL in H2O) |
| Растворимость в воде | 70 g/L (20 ºC) |
| Мерк | 14,7617 |
| Стабильность | Strong oxidizer - contact with combustible materials may cause fire. Incompatible with combustible material, organics, reducing agents, aluminium, finely powdered metals. |
| FDA 21 CFR | 172.730 |
| Вещества, добавляемые в пищу (ранее EAFUS) | POTASSIUM BROMATE |
| Справочник по базе данных CAS | 7758-01-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 6-9 |
| FDA UNII | 04MB35W6ZA |
| Предложение 65 Список | Potassium bromate |
| МАИР | 2B (Vol. Sup 7, 73) 1999 |
| Система регистрации веществ EPA | Potassium bromate (7758-01-2) |
| UNSPSC Code | 41116105 |
| NACRES | NB.24 |
| Коды опасности | O,T | |||||||||
| Заявления о рисках | 45-9-25-22 | |||||||||
| Заявления о безопасности | 53-45 | |||||||||
| РИДАДР | UN 1484 5.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | EF8725000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 5.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28299000 | |||||||||
| Банк данных об опасных веществах | 7758-01-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 157 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H350:Может вызывать раковые заболевания.
H271:Сильный окислитель; может вызвать возгорание или взрыв.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P301+P310+P330:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
Бромат калия химические свойства, назначение, производство
Химические свойства
Potassium bromate is a white crystalline solid.Использование
Potassium Bromate is a dough conditioner that exists as white crystals or powder and is soluble in water. it exists in the anhydrous form as white granular powder and in the hydrated form as small white crystals or granules. it is used to age and improve the baking properties of flour. it is used with potassium iodate and azodicarbon- amide to modify the protein in bread flour to promote the desired properties of loaf volume and shape. it is used in baked goods.Общее описание
A white crystalline solid.Реакции воздуха и воды
Water soluble.Профиль реактивности
Potassium bromate is a strong oxidizing agent. Forms very flammable mixtures with combustible materials. Such mixtures may be explosive if the combustible material is finely divided and are often ignitable by friction. A mixture with finely divided aluminum can explode by heat, percussion, and friction [Mellor 2:310. 1946-47]. Reacts explosively with selenium [Mellor 2 Supp1:763. 1956]. Prolonged exposure to fire or heat can result in an explosion .Опасность
Dangerous fire risk in contact with organic materials, strong irritant. Possible carcinogen.Угроза здоровью
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.Пожароопасность
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.Профиль безопасности
Confirmed carcinogen with experimental carcinogenic data. A poison by ingestion. A powerful oxidzer. An irritant to skin, eyes, and mucous membranes. Mutation data reported. Wxtures with sulfur may ignite. Violent reaction with Al, Al + dmitrotoluene @ 290°, As, C, Cu, Pb(C2H3O2)2, metal sulfides, organic matter, P, S. Aqueous solutions react violently with selenium. When heated to decomposition it emits very toxic fumes of Brand K2O. See also BROMIDES.Возможный контакт
Potassium bromate is used as animal feed additive, food additive; flavor and packaging material; as a laboratory reagent; an oxidizing agent.Перевозки
UN1479 Potassium bromate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.Методы очистки
Crystallise KBrO3 from distilled H2O (2mL/g) between 100o and 0o. To remove bromide contamination, a 5% solution in distilled H2O, cooled to 10o, is bubbled with gaseous chlorine for 2hours, then filtered and extracted with reagent grade CCl4 until colourless and odourless. After evaporating the aqueous phase to about half its volume, it is cooled again slowly to about 10o. The crystalline KBrO3 that separates, is washed with 95% EtOH and dried in a vacuum [Boyd et al. J Am Chem Soc 74 237 1952]. Another way to remove Br-ions is by stirring several times in MeOH and then drying at 150o [Field & Boyd J Phys Chem 89 3767 1985].Несовместимости
A strong oxidizer.Violent reaction with many compounds, including reducing agents; chemically active metals; combustible materials, strong acids, alkaline earth sulfides, aluminum carbides, aluminum, amines, calcium sulfide, carbides, chlorine trifluoride, glycerin, hydrides, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, hydroxylamine, magnesium, metal powders, metal sulfides, molybdenum, phenylhydrazine, phosphorous red/ friction, phosphorous trichloride, silicon, sulfides, sulfur, sulfur dioxide, sulfur/friction, sulfuric acid, tungsten, hydrogen trisulfide. Incompatible with aluminum, copper.Бромат калия запасные части и сырье
сырьё
1of4
запасной предмет
- 3-BROMO-2,4-DICHLORO-5-FLUORONITROBENZENE
- Деss-Marтин перioдиnane
- Benzilic acid
- 1-(2,4-DICHLORO-5-FLUORO-3-METHOXYPHENYL)ETHANONE
- 2-йодxyбензойная кислота
1of3
Бромат калия поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12815 | 58 | |
| +86-13131129325 | China | 5872 | 58 | |
| +86-18033737140 +86-17354101231 |
China | 227 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21630 | 55 | |
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29862 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8615060885618 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 |