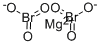
MAGNESIUM BROMATE
- русский язык имя
- английское имяMAGNESIUM BROMATE
- CAS №14519-17-6
- CBNumberCB7417890
- ФормулаBr2MgO6
- мольный вес280.11
- EINECS238-530-1
- номер MDLMFCD00049476
- файл Mol14519-17-6.mol
химическое свойство
| FDA UNII | 370X21564P |
| Система регистрации веществ EPA | Bromic acid, magnesium salt (14519-17-6) |
MAGNESIUM BROMATE химические свойства, назначение, производство
Описание
Magnesium bromate hexahydrate is a white crystalline compound that is insoluble in alcohol, but soluble in water. It reacts with methyl alcohol to form a complex. It is a fire hazard but is used as an analytical reagent.Magnesium bromate has found little usage in Industry because there are better oxidizing agents available. It is offered for sale commercially but in limited quantities.Химические свойства
White crystals or crystalline powder. Soluble in water; insoluble in alcohol.Использование
Analytical reagent, oxidizing agent.Подготовка
Magnesium Bromate can be prepared by the reaction of bromic acid and magnesium carbonate:MgCO3+ 2HBrO3→Mg(BrO3)2+ CO2+H2O
However, magnesium bromate is not stable in an acidic environment and tends to decompose if one attempts to remove it from solution. Thus, the best method of preparation involves the use of sodium bromate which is basic enough to prevent such decomposition in solution:
Mg(OH)2+ 2NaBrO3→Mg(BrO3)2+ 2NaOH
The product is a hexahydrate, Mg(BrO3)2·6H2O This salt can also be prepared by adding a solution of magnesium sulfate to a suspension of barium bromate:
MgSO4 (aq)+ Ba(BrO3)2 (s)→Mg(BrO3)2 (aq)+ BaSO4(s)
The solution is then evaporated to recrystallize the hexahydrate, Mg(BrO3)2·6H2O.
Общее описание
Crystalline solid. Soluble in water and denser than water. Hence sinks in water. Contact may cause irritation to skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.Реакции воздуха и воды
Soluble in water.Профиль реактивности
MAGNESIUM BROMATE is an oxidizing agent. May cause ignition in contact with organic materials. A combination of finely divided aluminum with finely divided MAGNESIUM BROMATE can explode by heat, percussion, or friction [Mellor 2:310. 1946-47].Опасность
Dangerous fire risk in contact with organic materials.Угроза здоровью
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.Пожароопасность
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.MAGNESIUM BROMATE запасные части и сырье
запасной предмет
MAGNESIUM BROMATE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 029-87576359 15353716720 |
China | 10009 | 58 | |
| 0751-2818792 13435114148 |
China | 2988 | 58 |