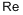
Рений
- английское имяRHENIUM
- CAS №7440-15-5
- CBNumberCB5179886
- ФормулаRe
- мольный вес186.21
- EINECS231-124-5
- номер MDLMFCD00011195
- файл Mol7440-15-5.mol
| Температура плавления | 3180 °C (lit.) |
| Температура кипения | 5596 °C (lit.) 5627 °C (lit.) |
| плотность | 21.02 g/cm3 (lit.) |
| температура хранения | Flammables area |
| растворимость | insoluble in HCl |
| форма | wire |
| цвет | Silver-gray |
| Удельный вес | 21.04 |
| РН | 5 (20°C in H2O) |
| Растворимость в воде | Insoluble in water. |
| Мерк | 13,8261 |
| Пределы воздействия | ACGIH: TWA 1 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 |
| ИнЧИКей | WUAPFZMCVAUBPE-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 7440-15-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 7YHU292INY |
| Система регистрации веществ EPA | Rhenium (7440-15-5) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | C,F,Xi | |||||||||
| Заявления о рисках | 34-11-36/38 | |||||||||
| Заявления о безопасности | 16-45-36/37/39-26-33-27 | |||||||||
| РИДАДР | UN 3178 4.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | VI0780000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 8112993090 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P234:Хранить только в оригинальной упаковке.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P363:Перед повторным использованием выстирать загрязненную одежду.
Рений химические свойства, назначение, производство
Химические свойства
Depending on the process used to isolate and process it, rhenium may appear as a brown-black powder or a silvery white solid metal. Rhenium is among the least common of the natural elements comprising 0.5–1 ppb of earth’s crust; it generally occurs as a trace element in molybdenite, columbite, gadolinite, and platinum ores. A sulfide mineral of rhenium, rhenite exists but is very rare.There are two naturally occurring isotopes, 185 (37%), which is stable, and 187 (63%), which has a halftime of 1011 years, and several synthetic radioisotopes whose half-lives range from ,<1 μs to 2×105 year. Rhenium has 11 valence states that range from 0 to 7.
Физические свойства
Rhenium ranges in color from silvery-white to gray to a black powder. It is a rather denseelement. As a refined metal, rhenium is ductile, but because it is rather rare, its properties havenot found many uses. Rhenium does have the widest range of valences. In addition to its commonvalences of 4, 6, and 7, it also has the uncommon valences of 2, –1, and –7.Rhenium has a high melting point of 3,180°C, a boiling point of 5,627°C, and a densityof 21.04 g/cm3.
Изотопы
There are 45 isotopes of rhenium. Only one of these is stable: Re-185, whichcontributes 37.40% to the total amount of rhenium found on Earth. Re-187, which isradioactive with a very long half-life of 4.35×10+10 years, contributes 62.60% to rhenium’sexistence on Earth. The remaining 43 isotopes are radioactive with relatively shorthalf-lives and are artificially manufactured.Происхождение имени
Derived from the Latin word Rhenus, which stands for the Rhine River in Western Europe.Вхождение
Rhenium is the 78th most common element found on Earth, which makes it somewhatrare. During the early twentieth century, it required the processing of about a 1,000 poundsof earth to secure just one pound of rhenium, resulting in a price of about $10,000 per gram.Thus, there were few uses for rhenium. Later in the century, improved mining and refiningtechniques reduced the price. Today, the United States produces about 1,000 pounds of rheniumper year, and the world’s total estimated supply is only about 400 tons.The main sources of rhenium are the molybdenite and columbite ores. Some rhenium isrecovered as a by-product of the smelting of copper sulfide (CuS) ores. Molybdenum sulfide(MoS2) is the main ore and is usually associated with igneous rocks and, at times, metallic-likedeposits. Molybdenite is found in Chile, as well as in the states of New Mexico, Utah, andColorado in the United States.
Характеристики
Rhenium is one of the transition elements, which range from metals to metal-like elements.Its chemical and physical properties are similar to those of technetium, which is aboveit in the periodic table. It is not very reactive. When small amounts are added to molybdenum,it forms a unique type of semiconducting metal. It is also noncorrosive in seawater.Использование
Small quantities of rhenium are alloyed with iron to form steel that is both hard and resistantto wear and high-temperatures. Because of its high melting point, rhenium is used inmany applications where long-wearing, high-temperature electrical components are required,such as electrical contacts and switches and high-temperature thermocouples. This physicalquality makes rhenium alloys ideal for use in rocket and missile engines. It is also used to formthe filaments in photographic flash lamps.Rhenium’s isotope (187Re) has a very long half-life and decays by both beta and alpharadiation at a very steady rate. This factor makes it useful as a standard to measure the age ofthe universe.
Определение
rhenium: Symbol Re. A silvery white metallic transition element;a.n. 75; r.a.m. 186.2; r.d. 20.53; m.p.3180°C; b.p. 5627 (estimated)°C. Theelement is obtained as a by-productin refining molybdenum, and is usedin certain alloys (e.g. rhenium–molybdenum alloys are superconducting).The element forms a numberof complexes with oxidationstates in the range 1–7. It was discoveredby Walter Noddack (1893–1960)and Ida Tacke (1896–1978) in 1925.Методы производства
Among the compounds that can be formed with rhenium are sulfides, fluorides, chlorides, bromides, iodides, and oxides. Rhenium(VII) oxide, Re2O7, is the most stable oxide of rhenium. It is formed from rhenium metal powder or other rhenium oxides in dry air or an oxygen atmosphere above 350° °C. Re2O7 is readily soluble in water, forming perrhenic acid, HReO4, which forms salts (MReO4) such as ammonium perrhenate (NH4ReO4). This is an important starting material, which can be reduced to Re metal and used for the production of many other rhenium compounds. Rhenium can also form organometallic compounds such as carbonyls (e.g., Re2(CO)10 and organorhenium compounds such as hexamethylrhenium, Re(CH3)6.Опасность
Rhenium is flammable in powder form. Rhenium dust and powder and many of its compoundsare toxic when inhaled or ingested.Промышленное использование
The outstanding properties of rhenium and rhenium alloys suggest their use in many specialized applications.Rhenium vs. tungsten thermocouples can be used for temperature measurement and control to approximately 2200 C, whereas previous thermocouple use was limited to temperatures below 1750 C. Indeed, 74% W 26% Re vs. W thermocouples can be used to temperatures of at least 2750 C with a high emf output ensuring accurate precise temperature measurements with excellent reproducibility and reliability.Rhenium is now widely used for filaments for mass spectrographs and for ion gauges for measuring high vacuum. Its ductility, chemical properties, including the fact that it does not react with carbon to form a carbide, and its emission characteristics make it superior to tungsten for these applications.
Rhenium has received considerable acclaim as an electrical contact material. It possesses excellent resistance to wear as well as arc erosion. Furthermore, the contact resistance of rhenium is extremely stable because of its good corrosion resistance in addition to the fact that possible formation of an oxide film on the contacts would not cause any appreciable change in the contact resistance since the resistivity of the oxide is almost the same as that of the metal. Extensive tests for some types of make-andbreak switching contacts has shown rhenium to have 20 times the life of platinum palladium contacts currently in use.
Рений запасные части и сырье
запасной предмет
Рений поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |