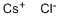
Цезия хлорид
- английское имяCesium chloride
- CAS №7647-17-8
- CBNumberCB6240178
- ФормулаClCs
- мольный вес168.36
- EINECS231-600-2
- номер MDLMFCD00010955
- файл Mol7647-17-8.mol
| Температура плавления | 645 °C (lit.) | ||||||||||||||
| Температура кипения | 1290 °C | ||||||||||||||
| Плотность накопления | 1800kg/m3 | ||||||||||||||
| плотность | 3.988 g/cm3 | ||||||||||||||
| плотность пара | 5.8 (vs air) | ||||||||||||||
| показатель преломления | 1.6418 | ||||||||||||||
| Fp | 1303°C | ||||||||||||||
| температура хранения | Store at +5°C to +30°C. | ||||||||||||||
| растворимость | H2O: 3 M at 20 °C, clear, colorless | ||||||||||||||
| форма | beads | ||||||||||||||
| Удельный вес | 3.988 | ||||||||||||||
| цвет | White | ||||||||||||||
| РН | 5.0-7.5 (25℃, 3M in H2O) | ||||||||||||||
| Запах | Odorless | ||||||||||||||
| Водородный показатель | 6.0 - 7.5 | ||||||||||||||
| Растворимость в воде | 1860 g/L (20 ºC) | ||||||||||||||
| Чувствительный | Hygroscopic | ||||||||||||||
| Кристальная структура | CsCl type | ||||||||||||||
| λмакс | λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.02 |
||||||||||||||
| crystal system | Cube | ||||||||||||||
| Мерк | 14,2011 | ||||||||||||||
| Space group | Pm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Стабильность | Stable. Deliquescent. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. | ||||||||||||||
| ИнЧИКей | AIYUHDOJVYHVIT-UHFFFAOYSA-M | ||||||||||||||
| Справочник по базе данных CAS | 7647-17-8(CAS DataBase Reference) | ||||||||||||||
| Рейтинг продуктов питания EWG | 1 | ||||||||||||||
| FDA UNII | GNR9HML8BA | ||||||||||||||
| Справочник по химии NIST | Cesium chloride(7647-17-8) | ||||||||||||||
| Система регистрации веществ EPA | Cesium chloride (CsCl) (7647-17-8) | ||||||||||||||
| Поглощение | ≤0.2 at 280 in H2O at 50% | ||||||||||||||
| UNSPSC Code | 12352302 | ||||||||||||||
| NACRES | NB.21 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 68-36/38 | |||||||||
| Заявления о безопасности | 36/37-26-24/25 | |||||||||
| РИДАДР | 2923 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | FK9625000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28273980 | |||||||||
| Банк данных об опасных веществах | 7647-17-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 i.p. in rats: 1.5 g/kg (Cochran) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H361f:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Цезия хлорид химические свойства, назначение, производство
Химические свойства
Cesium chloride (CsCl) is an inorganic, colorless, hygroscopic crystalline powder. It has a large mass and is highly soluble in water (1865 g/L). Due to its hygroscopic characteristic, when put in water, it forms a dense solute that is not very viscous. Therefore, it is a good material for equilibrium gradient differential centrifugation where the separation of the particles is size and density dependent.Cesium Chloride is a type of unit cell that is commonly mistaken as Body-Centered Cubic. This misconception is easy to make, since there is a center atom in the unit cell, but CsCl is really a non-closed packed structure type.
Использование
Cesium chloride has been used in density gradient centrifugation and for fractionation of nucleic acids, ribosomal subunits, proteins, glycoproteins, and viruses. Cesium chloride can also be used as a raw material to prepare other cesium compounds, such as cesium chloride reacts with nitric acid and oxalate to prepare cesium carbonate. It can be applied to the discharge tube (positive ion provided by the filament surface) fluorescent screen and used as a contrast agent. It effectively prevents activation of caspase-3 and neuronal apoptosis in serum- and potassium-deprived cerebellar granule neurons by inactivating GSK-3β.Подготовка
Cesium chloride (CsCl) is produced by the reaction of cesium metal with chlorine gas (Ca+ + Cl- → CsCl). It is also used in the beer brewing industry, to coat fluorescent screens, and to improve the taste of mineral water.Использование
Cesium chloride is used in radio and television vacuum tubes. It also is used in ultracentrifuge separations; x-ray fluorescent screens; as radiogrpahic contrast medium, and to prepare cesium and other cesium salts.Used for the preparation of electrically conducting glasses.
Used to make solutions for the separation of RNA from DNA by density gradient centrifugation.
For molecular genetic applications used in preparing nucleic acids for subsequent hybridization or cloning experiments.
Определение
ChEBI: Caesium chloride is the inorganic chloride salt of caesium; each caesium ion is coordinated by eight chlorine ions. It has a role as a phase-transfer catalyst and a vasoconstrictor agent. It is an inorganic chloride and an inorganic caesium salt.прикладной
Cesium chloride has been used in the generation of discontinuous gradient for the purification of Cryptosporidium oocysts and as a component of acetamide medium for fungal selection.Cesium chloride may be employed in gel-electrophoresis and RNA purification studies. It may be employed for the isolation of bacterial plasmid from Agrobacterium spp.
Общее описание
Cesium chloride is a cesium halide. Cesium halides can be prepared by reacting cesium carbonate with corresponding hydrohalic acids. Cesium chloride ultracentrifugation method has been reported for the extraction of RNA from cellular fractions.Угроза здоровью
Whereas conventional caesium chloride has a rather low toxicity to humans and animals, the radioactive form easily contaminates the environment due to the high solubility of CsCl in water. Spread of 137CsCl powder from a 93-gram container in 1987 in Goiania, Brazil, resulted in one of the worst-ever radiation spill accidents killing four and directly affecting 249 people.Профиль безопасности
Moderately toxic by ingestion and intraperitoneal routes. Experimental reproductive effects. Mutation data reported. Reacts violently with BF3. See also CESIUM. When heated to decomposition it emits toxic fumes of Cl-.Методы очистки
It is soluble in H2O but can be purified by crystallisation from H2O [solublity in g percent: 162.3(0.7o), 182.2(16.2o) and 290(at bp 119.4o)] and dried in high a vacuum. It is soluble in EtOH and is deliquescent; keep it in a tightly closed container. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 951-955 1963.] For further purification of CsCl, a concentrated aqueous solution of the practically pure reagent is treated with an equivalent weight of I2 and Cl2 is bubbled into the solution until preciptation of CsCl2I is complete. Recrystallisation yields a salt which is free from other alkali metals. It is then decomposed to pure CsCl on heating. [Harned & Schupp J Am Chem Soc 52 3886 1930.] It can also be recrystallised from acetone/water, or from water (0.5mL/g) by cooling in a CaCl2/ice bath. Dry it at 78o under vacuum.Цезия хлорид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617732866630 | China | 18147 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | ||
| +8615373025980 | China | 895 | 58 | ||
| +1-+1(833)-552-7181 | United States | 52924 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 |