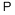
Фосфор
- английское имяPhosphorus
- CAS №7723-14-0
- CBNumberCB9238026
- ФормулаP
- мольный вес30.97
- EINECS231-768-7
- номер MDLMFCD00133771
- файл Mol7723-14-0.mol
| Температура плавления | 280 °C (white)(lit.) |
| Температура кипения | 280℃ |
| плотность | 2.34 g/mL at 25 °C(lit.) |
| Плотность накопления | 1100kg/m3 |
| плотность пара | 0.02 (vs air) |
| давление пара | 0.03 mm Hg ( 21 °C) |
| Fp | 30°C |
| температура хранения | 2-8°C |
| растворимость | insoluble |
| форма | powder (red) |
| цвет | Red-brown |
| Удельный вес | 2.34 |
| Запах | Acrid fumes when exposed to air |
| Flame Color | Pale blue-green |
| РН | 3 at 37℃ and 500-10000mg/L |
| удельное сопротивление | 10 μΩ-cm, 20°C |
| Растворимость в воде | insoluble |
| Мерк | 13,7433 |
| Пределы воздействия | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Диэлектрическая постоянная | 4.1(34℃) |
| Стабильность | Stable. Highly flammable. Incompatible with strong oxidizing agents, strong bases. Light and heat sensitive. |
| Справочник по базе данных CAS | 7723-14-0(CAS DataBase Reference) |
| FDA 21 CFR | 101.9; 104.20; 107.10; 107.100; 310.545 |
| Рейтинг продуктов питания EWG | 2-5 |
| Словарь онкологических терминов NCI | phosphorus |
| FDA UNII | 27YLU75U4W |
| Справочник по химии NIST | Phosphorus atom(7723-14-0) |
| Система регистрации веществ EPA | Phosphorus (7723-14-0) |
| UNSPSC Code | 12161700 |
| NACRES | NA.25 |
| Коды опасности | F,N,C,T+ | |||||||||
| Заявления о рисках | 11-16-52/53-50-35-26/28-17 | |||||||||
| Заявления о безопасности | 7-43-61-43C-45-38-26-5-27-6 | |||||||||
| РИДАДР | UN 1338 4.1/PG 3 | |||||||||
| OEB | D | |||||||||
| OEL | TWA: 0.1 mg/m3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | TH3495000 | |||||||||
| F | 10-21 | |||||||||
| Температура самовоспламенения | White phosphorus: 29 °C Red phosphorus: 260 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28047000 | |||||||||
| Банк данных об опасных веществах | 7723-14-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral (rat) 3 mg/kg PEL (OSHA) 0.1 mg/m3 TLV-TWA (ACGIH) 0.02 ppm (0.1 mg/m3) | |||||||||
| ИДЛА | 5 mg/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H228:Воспламеняющееся твердое вещество.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P370+P378:При пожаре тушить сухим песком, сухим химическим порошком или спиртостойкой пеной.
Фосфор химические свойства, назначение, производство
Описание
White or yellow white phosphorus is a yellow waxy or colourless, transparent, volatile crystalline solid, waxy appearance with a garlic-like odour. On exposure to light, it darkens and ignites in air. It is also called yellow phosphorus colour because of impurities. White phosphorus does not occur naturally but is manufactured from phosphate rocks. It is insoluble in water, slightly soluble in benzene, ethanol, and chloroform, and is soluble in carbon disulphide. White phosphorus reacts rapidly with oxygen, easily catching fire at temperatures 10°C–15°C above room temperature. White phosphorus is used by the military in various types of ammunition and to produce smoke for concealing troop movements and identifying targets. It is also used by industry to produce phosphoric acid and other chemicals for use in fertilisers, food additives, and cleaning compounds. Small amounts of white phosphorus were used in the past in pesticides and fireworks.White phosphorus is used mainly for producing phosphoric acid and other chemicals. These chemicals are used to make fertilisers, additives in foods and drinks, cleaning compounds, and other products. In the military, white phosphorus is used in ammunitions such as mortar and artillery shells, and grenades.Химические свойства
Yellow or white phosphorus ignites spontaneously in air at 34 °C. It should be stored under water. Under this condition, however, it may form phosphoric acid. Stainless steel containers should be used to hold the corrosive material. White phosphorus fires can be controlled by using water or sand or by excluding air.Изотопы
There are a 23 isotopes of phosphorus, ranging from P-24 to P-46, with halflivesthat range from a few nanoseconds to about two and half minutes. The one stableisotope is phosphorus-31, which accounts for 100% of the natural phosphorus on Earth.Происхождение имени
Its name is derived from the Greek word phosphoros, which means “bringer of light” or “light bearing.”Вхождение
Phosphorus is the 12th most abundant element. It makes up about 0.1% of the Earth’s crust.Phosphorous occurs in nature in several forms, mostly as phosphates. The most commonsource is phosphate rock [Ca3(PO4)2] and a mineral called “apatite.” Phosphorus is found inall animal bones and teeth and in most living tissue. Phosphorous nodules are found on theocean floor along with manganese nodules.Most commercial phosphorus is produced in electric furnaces where the phosphate-richminerals are heated to drive off the phosphorus as a gas, which is then condensed under water.Another process uses sulfuric acid to remove the phosphorus.Характеристики
White phosphorus occurs in nature in phosphate rock. It is insoluble in water and alcoholand will ignite spontaneously in air. It exhibits what is known as phosphorescence; that is, itglows in the dark at room temperature. White phosphorus is poisonous and must be storedunder water.Red phosphorus is less reactive than the white variety. It is not poisonous, but largeamounts can explode. It is used in fireworks and matches.
Black phosphorus is the only one of the three that will conduct electricity; white and redare poor conductors. Black phosphorus has no significant commercial uses.
Использование
It is used to make safety matches, incendiary shells,andsmokebombs;inpyrotechnics;and in the manufacture of fertilizers, pesticides, phosphoric acid, and phosphorus halides.Определение
phosphorus: Symbol P. A nonmetallicelement belonging togroup 15 (formerly VB) of the periodictable; a.n. 15; r.a.m. 30.9738; r.d.1.82 (white), 2.34 (red); m.p. 44.1°C(α-white); b.p. 280°C (α-white). It occursin various phosphate rocks,from which it is extracted by heatingwith carbon (coke) and silicon(IV)oxide in an electric furnace (1500°C).Calcium silicate and carbon monoxideare also produced. Phosphorushas a number of allotropic forms.The α-white form consists of P4 tetrahedra(there is also a β-white formstable below –77°C). If α-white phosphorusis dissolved in lead andheated at 500°C a violet form is obtained.Red phosphorus, which is acombination of violet and whitephosphorus, is obtained by heatingα-white phosphorus at 250°C with airexcluded. There is also a black allotrope,which has a graphite-likestructure, made by heating whitephosphorus at 300°C with a mercurycatalyst. The element is highly reactive.It forms metal phosphides andcovalently bonded phosphorus(III)and phosphorus(V) compounds. Phosphorusis an essential element forliving organisms. It is an importantconstituent of tissues (especiallybones and teeth) and of cells, beingrequired for the formation of nucleic acids and energy-carrying molecules(e.g. ATP) and also involved in variousmetabolic reactions. The elementwas discovered by Hennig Brand(c. 1630–92) in 1669.Общее описание
A white or yellow colored semi-liquid. Transported at high temperatures. Insoluble in water and denser than water. Contact may cause burns to skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. May ignite upon exposure to air. Used to make other chemicals.Реакции воздуха и воды
When exposed to air emits a green light and gives off white fumes. Ignites at 30°C in moist air, higher temperatures are required for ignition in dry air [Merck 11th ed. 1989]. The reactivity of phosphorus with oxygen or air depends on the allotrope of phosphorus involved and the conditions of contact, white (yellow) phosphorus being by far more reactive. White phosphorus readily ignites in air if warmed, finely divided, or under conditions where the slow oxidative isotherm cannot be dissipated. Contact with finely divided charcoal or lampblack promotes ignition, probably by the absorbed oxygen. Contact with amalgamated aluminum also promotes ignition [Mellor 1940 and 1971].Профиль реактивности
WHITE PHOSPHORUS reacts with air (fire, acidic solution); sulfur and oxidants (fire, explosion). Bromine trifluoride reacts similarly with arsenic, boron, bromine, iodine, phosphorus, and sulfur [Mellor 2:113. 1946-47]. Bromoazide explodes on contact with antimony, arsenic, phosphorus, silver foil, or sodium. Red phosphorus reacts in the cold with selenium oxychloride evolving light and heat; white phosphorus reacts explosively [Mellor 10:906. 1946-47]. When thorium is heated with phosphorus, they unite with incandescence [Svenska Akad. 1829. p. 1].Опасность
Many of the compounds of phosphorus are extremely dangerous, both as fire hazardsand as deadly poisons to the nervous system of humans and animals. Some of the poisonouscompounds (PClx) can be absorbed by the skin as well as inhaled or ingested. Flushing withwater is the only way to stop the burning of white phosphorus on the skin, but water doesnot affect the combustion of some phosphorus compounds. Although red phosphorus is notas dangerous or poisonous as white phosphorus, merely applying some frictional heating willinduce the red allotrope to change back to the explosive white allotrope (the striking of a safetymatch is an example).Some of the main types of poisonous gases used in warfare have a phosphorus base. Manycountries stockpile these gases, but, by agreement, the supplies are being reduced.
Угроза здоровью
White phosphorus is a highly toxic substance by all routes of exposure. Contact of the solid with the skin produces deep painful burns, and eye contact can cause severe damage. Ingestion of phosphorus leads (after a delay of a few hours) to symptoms including nausea, vomiting, belching, and severe abdominal pain. Apparent recovery may be followed by a recurrence of symptoms. Death may occur after ingestion of 50 to 100 mg due to circulatory, liver, and kidney effects. Phosphorus ignites and burns spontaneously when exposed to air, and the resulting vapors are highly irritating to the eyes and respiratory tract.Red phosphorus is much less toxic than the white allotrope; however, samples of red phosphorus may contain the white form as an impurity. Early signs of chronic systemic poisoning by phosphorus are reported to include anemia, loss of appetite, gastrointestinal distress, chronic cough, a garlic-like odor to the breath, and pallor. A common response to severe chronic poisoning is damage of the jaw (''phossy jaw") and other bones. Phosphorus has not been reported to show carcinogenic effects in humans.
Пожароопасность
White phosphorus ignites spontaneously upon contact with air, producing an irritating, dense white smoke of phosphorus oxides. Use water to extinguish phosphorus fires.Воспламеняемость и взрывоопасность
White phosphorus ignites spontaneously upon contact with air, producing an irritating, dense white smoke of phosphorus oxides. Use water to extinguish phosphorus fires.Red phosphorus is a flammable solid but does not ignite spontaneously on exposure to air. At high temperatures (-300 °C), red phosphorus is converted to the white form.
хранилище
Work with white phosphorus should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Phosphorus should be stored under water in secondary containers in areas separate from oxidizing agents and other incompatible substances.Методы очистки
Purify white phosphorus by melting it under dilute H2SO4—dichromate (possible carcinogen) mixture and allow to stand for several days in the dark at room temperature. It remains liquid, and the initial milky appearance due to insoluble, oxidisable material gradually disappears. The phosphorus can then be distilled under vacuum in the dark [Holmes Trans Faraday Soc 58 1916 1962]. It sublimes in vacuo. Other methods of purification include extraction with dry CS2 followed by evaporation of the solvent, or washing with 6M HNO3, then H2O, and drying under vacuum. It ignites in air at ~50o, or by friction if dry. Store and cut it under H2O . POISONOUS.Несовместимости
White phosphorus reacts with a number of substances to form explosive mixtures. For example, dangerous explosion hazards are produced upon reaction of phosphorus with many oxidizing agents, including chlorates, bromates, and many nitrates, with chlorine, bromine, peracids, organic peroxides, chromium trioxide, and potassium permanganate, with alkaline metal hydroxides (phosphine gas is liberated), and with sulfur, sulfuric acid, and many metals, including the alkali metals, copper, and iron.Red phosphorus is much less reactive than the white allotrope but may ignite or react explosively with strong oxidizing agents.
Утилизация отходов
Excess phosphorus and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.Фосфор запасные части и сырье
Фосфор поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 15369953316 +8615369953316 |
China | 2123 | 58 | ||
| +86-19355901310 +86-19355901310 |
China | 319 | 58 | ||
| +8617798174412 | China | 2097 | 58 | ||
| +1-3026880818 +1-3026880818 |
Canada | 66 | 58 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| 86-13657291602 | CHINA | 22963 | 58 | ||
| +8619521488211 | China | 2558 | 58 | ||
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | ||
| +8618523575427 | China | 49732 | 58 |