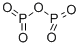
Фосфор пентоксид
- английское имяPhosphorus pentoxide
- CAS №1314-56-3
- CBNumberCB9245033
- ФормулаO5P2
- мольный вес141.94
- EINECS215-236-1
- номер MDLMFCD00011440
- файл Mol1314-56-3.mol
| Температура плавления | 340 °C (lit.) | ||||||||||||||
| Температура кипения | 122 °C (1 mmHg) | ||||||||||||||
| Плотность накопления | 700kg/m3 | ||||||||||||||
| плотность | 2.3 g/mL at 25 °C (lit.) | ||||||||||||||
| плотность пара | 4.9 (vs air) | ||||||||||||||
| давление пара | 1 mm Hg ( 384 °C) | ||||||||||||||
| показатель преломления | 1.433-1.436 | ||||||||||||||
| Fp | 340-360°C | ||||||||||||||
| температура хранения | no restrictions. | ||||||||||||||
| растворимость | Soluble in sulfuric acid. Insoluble in acetone and ammonia. | ||||||||||||||
| форма | Very Deliquescing Powder | ||||||||||||||
| Удельный вес | 2.39 | ||||||||||||||
| цвет | White | ||||||||||||||
| Запах | Pungent odour | ||||||||||||||
| Водородный показатель | <2 | ||||||||||||||
| РН | 1 (5g/l, H2O, 20℃) | ||||||||||||||
| Растворимость в воде | Soluble in sulfuric acid. Insoluble in acetone and ammonia. Decomposes in water. | ||||||||||||||
| Чувствительный | Moisture Sensitive | ||||||||||||||
| Мерк | 14,7355 | ||||||||||||||
| Сублимация | 340-360 ºC | ||||||||||||||
| crystal system | Three sides | ||||||||||||||
| Space group | R3c | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Стабильность | Stability Stable, but reacts violently with water, alcohols, metals, sodium, potassium, ammonia, oxidizing agents, HF, peroxides, magnesium, strong bases. | ||||||||||||||
| ИнЧИКей | YWEUIGNSBFLMFL-UHFFFAOYSA-N | ||||||||||||||
| Справочник по базе данных CAS | 1314-56-3(CAS DataBase Reference) | ||||||||||||||
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | PHOSPHOROUS PENTAOXIDE | ||||||||||||||
| Рейтинг продуктов питания EWG | 1 | ||||||||||||||
| FDA UNII | 51SWB7223J | ||||||||||||||
| Справочник по химии NIST | Diphosphorus pentoxide(1314-56-3) | ||||||||||||||
| Система регистрации веществ EPA | Phosphorus pentoxide (1314-56-3) | ||||||||||||||
| UNSPSC Code | 12352303 | ||||||||||||||
| NACRES | NB.21 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 35 | |||||||||
| Заявления о безопасности | 22-26-45 | |||||||||
| РИДАДР | UN 1807 8/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TH3945000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2809 10 00 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 1314-56-3(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P363:Перед повторным использованием выстирать загрязненную одежду.
Фосфор пентоксид химические свойства, назначение, производство
Химические свойства
Phosphorus pentoxide is a white crystalline solid.Физические свойства
White, deliquescent, powdery solid; exhibits polymorphism; converts to several different crystalline forms on heating; the commercial material consists of hexagonal crystals; the hexagonal crystals on very rapid heating first melt at 420°C and then resolidify immediately to glassy orthorhombic crystals; slow heating of hexagonal crystals causes melting at 340°C which, on solidification, gives the same metastable orthorhombic form; the glassy material melts at about 580°C to a colorless and heavily viscous liquid; sublimes at 360°C; density of the commercial product 2.39g/cm3; reacts with water.Использование
Phosphorus(V) oxide is used as a drying and dehydrating agent, a condensation reagent in organic synthesis and a laboratory reagent. It is also used in sugar refining and in fire extinguishing. Phosphorus pentoxide in DMSO forms an Onodera reagent which oxidizes alcohols. It is capable of converting mineral acids to anhydrides.Подготовка
Phosphorus pentoxide is prepared by burning phosphorus in a plentiful supply of dry air or oxygen: P4 + 5O2 → P4O10 The crude product may contain a small amount of sesquioxide, P2O3, which may be removed by sublimation in ozonized oxygen.Методы производства
Phosphorus pentoxide or phosphoric anhydride (P2O5) is formed by burning yellow phosphorus in dry air or oxygen.Общее описание
A white amorphous powder. Corrosive to metals and tissue and moderately toxic.Реакции воздуха и воды
Readily absorbs moisture from the air forming a syrup of meta-, pyro-, and orthophosphoric acids. Reacts violently with water releasing considerable heat [Oldbury Chemicals, p. 9].Профиль реактивности
Phosphorus pentoxide reacts violently and exothermically with water. The heat can ignite surrounding or admixed combustible materials. Undergoes hazardous or violent reactions with metal hydroxides and oxides, formic acid, hydrogen fluoride and hydrofluoric acid, iodides, metals (in particular potassium and sodium), oxidizing agents (bromine pentafluoride, chlorine trifluoride, perchloric acid, oxygen difluoride, hydrogen peroxide), ammonia, and proparygl alcohol. [Bretherick, 5th ed., 1995, p. 1781; EPA, 1998]. A violent explosion occurs if a solution of perchloric acid in chloroform is poured over phosphorus pentaoxide [EPA, 1998].Опасность
Phosphorus pentoxide is a strong irritant. It is corrosive to skin and contact with eyes can be injurious.Угроза здоровью
Because of its dehydrating action, phosphorus pentoxide is a highly corrosive substance. It is an irritant to the eyes, skin, and mucous membranes.Inhalation of its vapors caused chronic pulmonary edema, injury to lungs, and hemorrhage in test animals. The LC50 values varied significantly with the species.LC50 value, inhalation (rats): 127 mg/m3/h .
Пожароопасность
Reacts violently with water to evolve heat. Flammable poisonous gases may accumulate in tanks and hopper cars. Phosphorus pentoxide reacts violently with the following: ammonia, hydrofluoric acid, oxygen difluoride, potassium, sodium, propargyl alcohol, calcium oxide, sodium hydroxide and chlorine trifluoride. A violent explosion occurs if a solution of perchloric acid in chloroform is poured over phosphorus pentoxide. Avoid formic acid, hydrogen fluoride, inorganic bases, metals, oxidants, water. Readily absorbs moisture from air to form meta-, pryo-, or orthophosphoric acid.Сельскохозяйственное использование
Phosphoric anhydride is another name for phosphorus pentoxide (P2O5). Anhydrides react with water to give acids. For example, sulphur trioxide reacts with water to give sulphuric acid, or acetic anhydride reacts with water to give acetic acid. Similarly, phosphorus pentoxide has great affinity for water and dissolves in it to give phosphoric acid, and therefore, is known as phosphoric anhydride.Профиль безопасности
Poison by inhalation. A corrosive irritant to the eyes, shin, and mucous membranes. With the appropriate conditions it undergoes hazardous reactions with formic acid, hydrogen fluoride, inorganic bases, iodides, metals, methyl hydroperoxide, oxidants (e.g., bromine, pentafluoride, chlorine trifluoride, perchloric acid, oxygen Difluoride, hydrogen peroxide), 3-propynol, water. When heated to decomposition it emits toxic fumes of POx.Возможный контакт
This material is used as an intermediate in organic synthesis, catalyst, condensing agent; dehydrating agent; in the preparation of acrylate esters, surfactants, sugar refining; medicine, fire extinguishing; and special glasses.Методы очистки
It has been sublimed at 250o under vacuum into glass ampoules. It fumes in moist air and reacts violently with water. It is an excellent drying agent for use in desiccators. HARMFUL VAPOURS and attacks skin. [Manley J Chem Soc 121 331 1922, Klements in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 541 1963.]Несовместимости
Reacts violently and exothermically with water, forming ignition level heat and highly corrosive phosphoric acid. Keep away from the combination of moisture and combustible materials. Phosphorus pentoxide reacts violently with the following: perchloric acid; ammonia, hydrofluoric acid; oxidizers, hydrogen fluoride; formic acid, oxygen difluoride, potassium, sodium, propargyl alcohol; calcium oxide; inorganic bases; sodium hydroxide and chlorine trifluoride. Attacks many metals in presence of water. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alcohols, ammonia. Undergoes hazardous or violent reactions with metal hydroxides and oxides, formic acid, hydrogen fluoride and hydrofluoric acid, iodides, metals (in particular potassium and sodium), ammonia, and proparygl alcohol.Утилизация отходов
Decompose with water, forming phosphoric and hydrochloric acids. The acids may then be neutralized and diluted slowly to solution of soda ash and slaked lime with stirring then flush to sewer with large volumes of water.Фосфор пентоксид запасные части и сырье
сырьё
запасной предмет
- Petroleum additive
- Пиридоксаль фосфат
- BIS(2-THIENYL) KETONE
- 4-AMINO-2-CHLORO-6-METHYL-5-NITROPYRIMIDINE
- трет-Бутилкарбамидин гидрохлорид
- antistatic Agent P
- 2,5-BIS(3-METHOXYPHENYL)-1,3,4-OXADIAZOLE
- Amitriptyline hydrochloride
- 4-METHYLUMBELLIFERYL PHOSPHATE
- 2,5-дифенил-1 ,3,4-оксадиазол
- 5-Nitro-6-methyluracil
- 3-аминоизоксазол
- 2-Хлор-5-(хлорметил) пиридина
- Ammonium polyphosphate
- Этил 4-бромкротона
- Hexafluoroglutaric ангидрид
- Дифосфоrил хлорид
- 1-AMINO-N-HYDROXY-2,2-DIMETHYLPROPAN-1-AMINE
- 4-хроманон
- 7-Bromoisoquinoline
- Bis(4-nitrophenyl) phosphate
- Antistatic agent PK
- 5-Ethoxy-4-methyloxazole
- Гептафтормасляной ангидрид
- Триметилацетамид
- Трихлорацетонитрил
- (-)-MENTHYL CHLORIDE
- 2,5-БИС (3-НИТРОФЕНИЛ) -1,3,4-ОКСАДИАЗОЛ
- 2,4-Dichloro-6-methyl-5-nitropyrimidine
- 2,5-Бис-(1-нафтил) -1,3,4-оксадиазол
1of8
Фосфор пентоксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13637972665 +86-13637972665 |
China | 81 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| 86-21-65208861- 8007 | CHINA | 117 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |