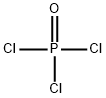
ОКСИХЛОРИД ФОСФОРА
- английское имяPhosphorus oxychloride
- CAS №10025-87-3
- CBNumberCB5218244
- ФормулаCl3OP
- мольный вес153.33
- EINECS233-046-7
- номер MDLMFCD00011443
- файл Mol10025-87-3.mol
| Температура плавления | 1.25 °C(lit.) |
| Температура кипения | 107 °C |
| плотность | 1.645 g/mL at 25 °C(lit.) |
| плотность пара | 5.3 (vs air) |
| давление пара | 104 mm Hg ( 50 °C) |
| показатель преломления | n |
| Fp | 105.8°C |
| температура хранения | Store below +30°C. |
| растворимость | Phosphorus(V) oxychloride is soluble in many organic solvents. |
| форма | Liquid |
| Удельный вес | 1.692 (15/15℃) |
| цвет | Colorless |
| Запах | Pungent odour |
| РН | 1.0 (5g/l, H2O, 25℃) |
| Растворимость в воде | reacts exothermically |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,7349 |
| Пределы воздействия | TLV-TWA0.628 mg/m3 (0.1 ppm)(ACGIH). |
| Диэлектрическая постоянная | 14.0(22℃) |
| Стабильность | Stable. Reacts violently with water. Incompatible with many metals, alcohols, amines, phenol, DMSO, strong bases. |
| ИнЧИКей | XHXFXVLFKHQFAL-UHFFFAOYSA-N |
| LogP | 0.357 (est) |
| Вещества, добавляемые в пищу (ранее EAFUS) | PHOSPHORUS OXYCHLORIDE |
| Справочник по базе данных CAS | 10025-87-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | 9XM78OL22K |
| Справочник по химии NIST | Phosphoryl chloride(10025-87-3) |
| Система регистрации веществ EPA | Phosphorus oxychloride (10025-87-3) |
| UNSPSC Code | 12352300 |
| NACRES | NA.22 |
| Коды опасности | T+,C | |||||||||
| Заявления о рисках | 14-22-26-29-35-48/23-25 | |||||||||
| Заявления о безопасности | 26-45-7/8-36/37/39 | |||||||||
| РИДАДР | UN 1810 8/PG 2 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.1 ppm (0.6 mg/m3), STEL: 0.5 ppm (3 mg/m3) | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TH4897000 | |||||||||
| F | 19-21 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28121020 | |||||||||
| Банк данных об опасных веществах | 10025-87-3(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H330:Смертельно при вдыхании.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P314:В случае плохого самочувствия обратиться к врачу.
ОКСИХЛОРИД ФОСФОРА химические свойства, назначение, производство
Химические свойства
Phosphorus oxychloride is a clear, colorless to yellow, fuming, oily liquid with a pungent and musty odor.Физические свойства
Colorless fuming liquid with a pungent odor; density 1.645 g/mL; freezes at 1°C; boils at 105.5°C; reacts with water and ethanol.Использование
Phosphorus oxychloride is an important intermediate in the production of triarylphosphate esters (e.g., triphenyl phosphate and tricresyl phosphate), which have been used as flame retardants and plasticizers for PVC. It is acutely toxic to the eyes, throat, and respiratory tract. Phosphorus oxychloride is also used in nuclear reprocessing, as chlorinating agent, especially to replace oxygen in organic compounds, as solvent in cryoscopy and the semiconductor industry.Подготовка
Phosphorus oxychloride can be prepared from phosphorus trichloride or phosphorus pentachloride. It can be obtained from phosphorus trichloride by cautious addition of potassium chlorate:3PCl3 + KClO3 → 3POCl3 + KCl The oxychloride also is obtained by the action of boric acid or oxalic acid with phosphorus pentachloride: 3PCl5 + 2B(OH)3 → 3POCl3 + B2O3 + 6HCl PCl5 + (COOH)2 → POCl3 + CO + CO2 + 2HCl Phosphorus oxychloride also is made by heating calcium phosphate in a current of chlorine and carbon monoxide at 350°C: 2Ca3(PO4)2 + 9Cl2 + 6CO → 4POCl3 + 6CaCO3 Alternatively, heating a mixture of calcium phosphate and carbon in a current of chlorine at 750°C yields the oxychloride.Определение
A white crystalline solid. It is a monobasic acid forming the anion H2PO2 – in water. The sodium salt, and hence the acid, can be prepared by heating yellow phosphorus with sodium hydroxide solution. The free acid and its salts are powerful reducing agents.Профиль реактивности
Phosphorus oxychloride is water reactive. Incompatible with strong oxidizing agents, alcohols, bases (including amines). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Combining the chloride with zinc dust caused immediate ignition, due to the formation of phosphine gas which ignites, [Mellor, 1940, Vol. 8, 1025]. An exotherm starting with the mixing of Phosphorus oxychloride with acetone (a ketone) lead to an explosion, may behave similarly with other ketones, [Organic Process Research and Development, Vol.4, No. 6,200, "Phosphorus oxychloride and Acetone: An Incompatibility Investigation Using ARC."]Опасность
The compound is highly irritating to skin, eyes and mucous membranes. Inhaling its vapors can cause pulmonary edema.Угроза здоровью
Inhalation of vapors of phosphorus oxychloride produced acute and chronic toxicity in test subjects. In humans, exposure to its vapors may cause headache, dizziness, weakness, nausea, vomiting, coughing, chest pain, bronchitis, and pulmonary edema. Most of these symptoms are manifested from chronic exposure to its vapors.LC50 value, inhalation (rats): 48 ppm (301 mg/m3)/4 h
Vapors of this compound are an irritant to the eyes and mucous membranes. The liquidis corrosive and can cause skin burns. An oral LD50 value for rats is documented to be 380 mg/kg (NIOSH 1986)..
Пожароопасность
Poisonous, corrosive, and irritating gases are generated when Phosphorus oxychloride is heated or is in contact with water. Phosphorus oxychloride may ignite other combustible materials (wood, paper, oil, etc.). Phosphorus oxychloride reacts violently with water. When heated to decomposition, Phosphorus oxychloride emits toxic fumes of chlorides and oxides of phosphorus; Phosphorus oxychloride will react with water or steam to produce heat and toxic and corrosive fumes. Incompatible with carbon disulfide; N,N-dimethylformamide; 2,5-dimethylpyrrole; 2,6-dimethyl- pyridine N-oxide; dimethylsulfoxide; Ferrocene-1,1-dicarboxylic acid; water; and zinc. Do not store with combustible materials, particularly fibrous organic materials, or with electrical or other equipment that can be corroded. Reacts violently with moisture.Возможный контакт
Phosphorus oxychloride is used in the manufacture of pesticides, pharmaceuticals, plasticizers, gasoline additives; and hydraulic fluids.Перевозки
UN1810 Phosphorus oxychloride, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Hazard Zone B.Методы очистки
Distil the liquid under reduced pressure to separate it from the bulk of the HCl and the phosphoric acid (from hydrolysis); the middle fraction is re-distilled into ampoules containing a little purified mercury. These ampoules are sealed and stored in the dark for 4-6weeks with occasional shaking to facilitate reaction of any free chloride with the mercury. The POCl3 is then again fractionally distilled and stored in sealed ampoules in the dark until required [Herber J Am Chem Soc 82 792 1960]. Lewis and Sowerby [J Chem Soc 336 1957] refluxed their distilled POCl3 with Na wire for 4hours, then removed the Na and again distilled. Use Na only with almost pure POCl3 to avoid explosions. HARMFUL VAPOURS; work in an efficient fume cupboard.Несовместимости
A powerful oxidizer. Violently decomposes in water, forming heat and hydrochloric and phosphoric acids. Violent reaction with alcohols, phenols, amines, reducing agents; combustible materials; carbon disulfide; dimethylformamide, and many other many materials. Rapid corrosion of metals, except nickel and lead.Утилизация отходов
Pour onto sodium bicarbonate. Spray with aqueous ammonia and add crushed ice. Neutralize and pour into drain with running water. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.ОКСИХЛОРИД ФОСФОРА запасные части и сырье
сырьё
1of2
запасной предмет
- 2,4-DICHLOROTHIENO[3,2-D]PYRIMIDINE
- DICAPTHON
- 2,2'-БИТИОФЕН-5-КАРБОКСАЛЬДЕГИД
- 5-METHYL-2-METHYLSULFANYL-PYRIMIDINE
- Бензальдегид азотистый горчица
- 2-Trifluoromethylbenzylsulfonyl chloride
- 4,6-дихлор-5-метоксипиримидина
- 2-ХЛОРО-4-МЕТИЛХИНОЛИН-3-КАРБОНИТРИЛ
- 4-(4-(BIS-(2-CHLOROETHYL)AMINO)BENZYLIDENE-2-PHENYL-OXAZOLINE-5-ONE
- Hexafluoroglutaric ангидрид
- 6-N-ГЕКСИЛАМИНОПУРИН
- 2,4-Dichlorothieno[3,2-d]pyrimidine
- Крахмал, гидрофосфат, 2-гидроксипропиловый эфир
- 2,6-Дихлор-4,8-дипиперидинопиримидино[5,4-d]пиримидин
- 2,4-дихлор-5-нитропиримидин
- 4-нитрофенил фосфат, динатриевая соль, гексагидрат
- 4-ТЕРТ-БУТИЛФЕНИЛОВЫЙ ЭФИР салициловой кислоты
- Трис(2-этилгексил)фосфат
- 2-этокси-1-нафтальдегид
- Хлорамбуцил
- 4-Хлор-3,5-динитробензальдегид
- 6-CHLORO-5-CYANO-4-METHOXYMETHYL-3-NITRO-2-PICOLINE
- 2-хлор-3 ', 4'-дигидроксиацетофенон
- 2,4,6-трихлорпиридин
- DISTARCH PHOSPHATE
1of8
ОКСИХЛОРИД ФОСФОРА поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +8615387054039 | China | 427 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| 18017061086 | CHINA | 5600 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 |