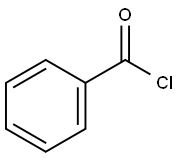
Бензоилхлорид
- английское имяBenzoyl chloride
- CAS №98-88-4
- CBNumberCB8854753
- ФормулаC7H5ClO
- мольный вес140.57
- EINECS202-710-8
- номер MDLMFCD00000653
- файл Mol98-88-4.mol
| Температура плавления | -1 °C (lit.) |
| Температура кипения | 198 °C (lit.) |
| плотность | 1.211 g/mL at 25 °C (lit.) |
| плотность пара | 4.88 (vs air) |
| давление пара | 1 mm Hg ( 32 °C) |
| показатель преломления | n |
| Fp | 156 °F |
| температура хранения | Store below +30°C. |
| растворимость | Acetonitrile (Slightly), Chloroform (Sparingly) |
| форма | Liquid |
| цвет | Clear |
| РН | 2 (1g/l, H2O, 20℃) |
| Запах | Pungent characteristic. |
| Водородный показатель | 2 at 1 g/l |
| Пределы взрываемости | 2.5-27%(V) |
| Растворимость в воде | reacts |
| Точка замерзания | -1℃ |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,1112 |
| БРН | 471389 |
| Диэлектрическая постоянная | 23.0(0℃) |
| Пределы воздействия | ACGIH: Ceiling 0.5 ppm |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, water, alcohols, strong bases. Reacts violently with DMSO and vigorously with alkalies. |
| ИнЧИКей | PASDCCFISLVPSO-UHFFFAOYSA-N |
| LogP | 1.44 at 21℃ and pH6 |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | BENZOYL CHLORIDE |
| Справочник по базе данных CAS | 98-88-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4 |
| FDA UNII | VTY8706W36 |
| МАИР | 2A (Vol. 29, Sup 7, 71) 1999 |
| Справочник по химии NIST | Benzoyl chloride(98-88-4) |
| Система регистрации веществ EPA | Benzoyl chloride (98-88-4) |
| UNSPSC Code | 12352106 |
| NACRES | NA.22 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 34-43-20/21/22 | |||||||||
| Заявления о безопасности | 26-45-36/37/39 | |||||||||
| РИДАДР | UN 1736 8/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | DM6600000 | |||||||||
| Температура самовоспламенения | 600 °C | |||||||||
| Примечание об опасности | Corrosive | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29310095 | |||||||||
| Банк данных об опасных веществах | 98-88-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2460 mg/kg LD50 dermal Rabbit 790 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H302+H312:Вредно при проглатывании или при попадании на кожу.
H331:Токсично при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Бензоилхлорид химические свойства, назначение, производство
Химические свойства
Benzoyl chloride is a colorless to slight brown liquid with a strong, penetrating odor; vapor causes tears. Soluble in ether and carbon disulfide; decomposes in water. Combustible. It is a liquid acyl chloride used as a benzoylating agent.Методы производства
Benzoyl chloride can be prepared from benzoic acid by reaction with PCl5 or SOCl2, from benzaldehyde by treatment with POCl3 or SO2 Cl2, from benzotrichloride by partial hydrolysis in the presence of H2SO4 or FeCl3, from benzal chloride by treatment with oxygen in a radical source, and from several other miscellaneous reactions. Benzoyl chloride can be reduced to benzaldehyde, oxidized to benzoyl peroxide, chlorinated to chlorobenzoyl chloride and sulfonated to m-sulfobenzoic acid. It will undergo various reactions with organic reagents. For example, it will add across an unsaturated (alkene or alkyne) bond in the presence of a catalyst to give the phenylchloroketone: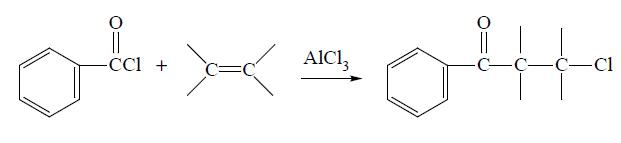
Определение
ChEBI: Benzoyl chloride is an acyl chloride consisting of benzene in which a hydrogen is replaced by an acyl chloride group. It is an important chemical intermediate for the manufacture of other chemicals, dyes, perfumes, herbicides and pharmaceuticals. It has a role as a carcinogenic agent. It is an acyl chloride and a member of benzenes. It is functionally related to a benzoic acid.?Общее описание
Benzoyl chloride appears as a colorless fuming liquid with a pungent odor. Flash point 162 °F. Lachrymator, irritating to skin and eyes. Corrosive to metals and tissue. Density 10.2 lb / gal. Used in medicine and in the manufacture of other chemicals.Профиль реактивности
Benzoyl chloride reacts violently with protic solvents such as alcohols, with amines and amides (for example dimethylformamide [Bretherick 1979 p. 6] ) and with inorganic bases. Causes the violent decomposition of dimethyl sulfoxide [Chem. Eng. News 35(9): 87 1957]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Friedel-Crafts acylation of naphthalene using Benzoyl chloride, catalyzed by AlCl3, must be conducted above the melting point of the mixture, or the reaction may be violent [Clar, E. et al., Tetrahedron, 1974, 30, 3296].Опасность
Highly toxic. Strong irritant to skin, eyes, and mucous membranes, and via ingestion, inhala- tion. Upper respiratory tract irritant. Probable car- cinogen.Угроза здоровью
INHALATION: may irritate eyes, nose and throat. INGESTION: causes acute discomfort. SKIN: causes irritation and burning.Химическая реактивность
Reactivity with Water Slow reaction with water to produce hydrochloric acid fumes. The reaction is more rapid with steam; Reactivity with Common Materials: Slow corrosion of metals but no immediate danger; Stability During Transport: Not pertinent; Neutralizing Agents for Acids and Caustics: Soda ash and water, lime; Polymerization: Does not occur; Inhibitor of Polymerization: Not pertinent.Механизм действия
Indicative of its high reactivity (relative to alkyl chlorides), benzyl chloride reacts with water in a hydrolysis reaction to form benzyl alcohol and hydrochloric acid. In contact with mucous membranes, hydrolysis produces hydrochloric acid. Thus, benzyl chloride is a lachrymator and has been used in chemical warfare. Theoretically, for every mole of benzyl chloride reacted, one mole of hydrochloric acid is released[1].Токсикология
Benzoyl chloride is of low acute oral toxicity in rats (LD50 2529 mg/kg). It is more toxic by inhalation (LC50 230 ppm, 4 h in male rats and 314 ppm, 4 h in female rats). The compound is irritating to skin, mucous membranes, eyes, and the respiratory tract.When benzoyl chloride or solutions of benzoyl chloride in benzene were applied to the skin of mice for up to 10 months irritation and keratinization resulted, and to some extent, ulceration and necrosis of the skin occurred. A few tumors (skin, lung) were observed in those mice. There is no clear evidence that benzoyl chloride is mutagenic.
For humans, benzoyl chloride is classified as a lachrymator. It is irritating to the skin, eyes, and mucous membranes. The available data are inadequate to evaluate the carcinogenic potential of benzoyl chloride to humans.
Возможный контакт
Benzoyl chloride is used as a chemical intermediate; in organic synthesis; to produce other chemicals, dyes, perfumes, herbicides, and medicines.Перевозки
UN 1736 Benzoylchloride, Hazard class: 8; Labels: 8—Corrosive material.Методы очистки
A solution of benzoyl chloride (300mL) in *C6H6 (200mL) is washed with two 100mL portions of cold 5% NaHCO3 solution, separated, dried with CaCl2 and distilled [Oakwood & Weisgerber Org Synth III 113 1955]. Repeated fractional distillation at 4mm Hg through a glass helices-packed column (avoiding porous porcelain or silicon-carbide boiling chips, and hydrocarbon or silicon greases on the ground joints) gave benzoyl chloride that did not darken on addition of AlCl3. Further purification is achieved by adding 3 mole% each of AlCl3 and toluene, standing overnight, and distilling off the benzoyl chloride at 1-2mm [Brown & Jenzen J Am Chem Soc 80 2291 1958]. Refluxing for 2hours with an equal weight of thionyl chloride before distillation has also been used. [Beilstein 9 IV 721.] Strong IRRITANT. Use in a fume cupboard.Несовместимости
May form explosive mixture with air. Contact with heat, hot surfaces, and flames causes decomposition, forming phosgene and hydrogen chloride. Water contact may be violent; forms hydrochloric acid. Reactions with amines, alcohols, alkali metals, dimethyl sulfoxide, strong oxidizers, and metal salts may be violent, causing fire and explosions. Attacks metals in the presence of moisture, forming explosive hydrogen gas. Attacks some plastics, rubber or coatings.Утилизация отходов
Pour into sodium bicarbonate solution and flush to sewer.Бензоилхлорид запасные части и сырье
сырьё
1of2
запасной предмет
- 3-oxo-3-phenyl-propanamide
- N,N-диметилпиперидин-4-амин
- APLPHA-BROMO-M-BENZOYLOXYACETOPHENONE
- 2- (N, N-ДИЭТИЛАМИНОКАРБОНИЛ) ФЕНИЛБОРОНОВАЯ КИСЛОТА
- 2-Метокси-3-метил-2-фенил-4H-бензо-g-пиранон
- (+)-Дибензоил-D-винной кислоты
- ДИБЕНЗОЙЛ ТИАМИН
- PHENOXYACETIC ACID
- Benzoyl cyanide
- 2,5-ДИХЛОРБЕНЗОЙЛ ХЛОРИД
- 3,4-диаминобензофенон
- Sucrose benzoate
- 1,3-Dibenzoyloxybenzene
- Хинолин-2-карбонитрил
- 1-Benzoylnaphthalene
- трет-Бутилпероксибензоат
- 3,4-дихлорбензофенона
- Быстрый синий BB
- dihydroxyethyl p-octadecyl phenylsulfonyl amino propyl ammoium propylsulfonate
- 3-хлорбензоил хлорид
1of8
Бензоилхлорид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617320528784 | China | 42 | 58 | ||
| +86-025-52279164 +8615996340606 |
China | 79 | 58 | ||
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 | ||
| +86-0523-86392777 +86-19825580222 |
China | 23 | 58 | ||
| +86-025-86655873 +8613962173137 |
China | 195 | 55 | ||
| +86-15531157085; +8615531157085 |
China | 8805 | 58 | ||
| +86-13131129325 | China | 5471 | 58 | ||
| +86-510-82753588 +86-13806194144 |
China | 300 | 58 | ||
| +86-15790943001 +86-15790943001 |
China | 374 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29730 | 60 |
Бензоилхлорид Обзор)
1of4