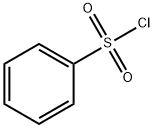
Бензолсульфонилхлорид
- английское имяBenzenesulfonyl chloride
- CAS №98-09-9
- CBNumberCB6854744
- ФормулаC6H5ClO2S
- мольный вес176.62
- EINECS202-636-6
- номер MDLMFCD00007426
- файл Mol98-09-9.mol
| Температура плавления | 13-15 °C (lit.) |
| Температура кипения | 251-252 °C (lit.) |
| плотность | 1.384 g/mL at 25 °C (lit.) |
| давление пара | 0.04 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | >230 °F |
| температура хранения | Store below +30°C. |
| растворимость | alcohol: soluble |
| форма | Liquid |
| цвет | Clear |
| Растворимость в воде | may decompose |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,1072 |
| БРН | 606926 |
| Стабильность | Stable. Incompatible with water, strong oxidizing agents, strong bases, methyl formamide, dimethyl sulfoxide. |
| LogP | 3.39 at 23℃ |
| Справочник по базе данных CAS | 98-09-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | OI9V0QJV9N |
| Справочник по химии NIST | Benzenesulfonyl chloride(98-09-9) |
| Система регистрации веществ EPA | Benzenesulfonyl chloride (98-09-9) |
| UNSPSC Code | 12352106 |
| NACRES | NA.22 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 20/22-34-42/43-29-22 | |||||||||
| Заявления о безопасности | 23-26-36/37/39-45 | |||||||||
| РИДАДР | UN 2225 8/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | DB8750000 | |||||||||
| F | 21 | |||||||||
| Температура самовоспламенения | 450°C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29049090 | |||||||||
| Банк данных об опасных веществах | 98-09-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orl-rat: 1960 mg/kg MEPAAX 20,513,69 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Бензолсульфонилхлорид химические свойства, назначение, производство
Химические свойства
Benzenesulfonyl chloride is a colorless oily liquid with a pungent odor. Insoluble in water, soluble in ethanol and ether. Can react with ammonia, amine and alcohol to produce benzenesulfonamide and benzene sulfonate respectively. It is toxic, irritates skin, eyes and mucous membranes, corrosive, and can cause burns. Oral LD50 1960mg/kg in rats.Использование
Benzenesulfonyl chloride reacts with Grignard reagents to form oxindoles from N-unsubstituted indoles. It is widely used to check the assay of thiamine in different food products. It is involved in the synthesis of alpha-disulfones, sulfonamides and sulfoante esters as precursor. It is a derivatization reagent for the determination of various amines in waste water and surface water at the sub-ppb level by gas chromatography-mass spectrometry. It is common reagent used in Hinsberg test for detection and distinguishing the type of amines as primary, secondary and tertiary amines.Методы производства
The most convenient method for the production of benzenesulfonyl chlorides is the chlorosulfonation reaction of benzene or substituted benzene with chlorosulfuric acid. Sulfonation and formation of the sulfonyl chlo- ride take place in one reaction sequence:Ar-H+Cl-SO3H→ArSO3H+HCl
ArSO3H+Cl-SO3H=ArSO2Cl+H2SO4
Sulfone is formed in a side reaction of the chlorosulfonation. The amount of sulfone formation can be reduced by diluting with a solvent, by using a large excess of chlorosulfuric acid, or by adding sulfone-inhibiting substances, e.g., alkali metal and ammonium salts, acetic acid, phosphoric acid, dimethylformamide, or amidosulfuric acid.
For industrial chlorosulfonation the chlorosulfuric acid is introduced into a cast-steel or enameled steel vessel and 10 – 25 mol% of the aromatic compound is stirred in at 25 – 30 ℃, whereupon sulfonation of the aromatic compound and HCl formation occur. The formation of sulfonyl chloride is initiated by heating the reactants to 50 – 80 ℃. The reaction is exothermic. The sulfonyl chloride is isolated by draining the reaction mass onto water and simultaneous cooling. Excess chlorosulfuric acid is decomposed, and the sulfonyl chloride either precipitates or separates as an organic liquid phase. The quality of the chlorosulfuric acid affects the yield.
Other chlorinating agents, such as phosgene, thionyl chloride, sulfuryl chloride, or phosphorus pentachloride, can be used instead of chlorosulfu- ricacid. When thionyl chloride is used the sulfonyl chloride is obtained from the sulfonic acid in high yield and without formation of sulfuric acid.
Общее описание
A colorless to slightly yellow solid that melts at approximately 40°F. Very irritating to skin, eyes and mucous membranes. May emit toxic fumes when heated to high temperatures. Used to make dyes and other chemicals.Реакции воздуха и воды
Insoluble and stable in cold water [Merck]. Decomposes in hot water to produce corrosive and toxic hydrochloric acid and benzenesulfonic acid. Rate of reaction decreases as temperature decreases.Профиль реактивности
Benzenesulfonyl chloride is incompatible with strong oxidizing agents and bases, including amines. Corrodes metals in the presence of water due to slow formation of hydrochloric acid and benzenesulfonic acid [USCG, 1999]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].Угроза здоровью
May be fatal if inhaled, swallowed or absorbed through skin. Contact may cause skin and eye burns. Irritating to eyes, skin and mucous membranes. INGESTION: May cause abdominal spasm and vomiting.Профиль безопасности
Poison by intraperitoneal route. Adangerous storage hazard. It may explode in a sealedbottle. Explosive reaction with dimethyl sulfoxide. Reactsvigorously with methyl formamide. When heated todecomposition it emits toxic fumes of Cl- and SOВозможный контакт
It is used as a chemical intermediate for benzenesulfonamides, thiophenol, glybuzole (hypoglycemic agent), N-2-chloroehtylamides, benzonitrile; for its esters-useful as insecticides, and miticides.Перевозки
UN2225 Benzene sulfonyl chloride, Hazard class: 8; Labels: 8—Corrosive material.Методы очистки
Distil the sulfonyl chloride, then treat it with 3mole % each of toluene and AlCl3, and allow it to stand overnight. The sulfonyl chloride is distilled off at 1mm pressure and then carefully fractionally distilled at 10mm in an all-glass column. [Adams & Marvel Org Synth Coll Vol I 84 1941, Jensen & Brown J Am Chem Soc 80 4042 1958, Beilstein 11 IV 49.] It is TOXIC.Несовместимости
Violent reaction with strong oxidizers, dimethyl sulfoxide, and methyl formamide. It is very reactive with bases and many organic compounds. Incompatible with ammonia, aliphatic amines. Water contact forms hydrochloric and chlorosulfonic acids. Aqueous solutions of this chemical are strong acids that react violently with bases. Attacks metals in presence of moisture.Бензолсульфонилхлорид запасные части и сырье
сырьё
запасной предмет
- 2-(2,3-DIHYDRO-1BENZENESULFONYL-PYRROLO[2,3-B]PYRIDIN-3-YL)ETHANAMINE
- Сульфасалазин
- Azoic Diazo Component 34
- (трифторметил)триметилсилан
- 17-гидроксипрегна-4,9(11)-диен-3,20-дион
- 2-(2-CHLOROETHOXY)-BENZENESULFONAMIDE
- Dibenzenesulfonamide
- Сульфахиноксалин
- Тиофенол
- 16alpha,17-epoxypregna-4,9(11)-diene-3,20-dione
- Бензолсульфонамид
- 2-Метил-3-нитроанилина
- Бензолсульфонил гидразид
- BENZENETHIONOSULFONIC ACID, SODIUM SALT
- Edifenphos
- 2,4-Дихлор-5-sulfamoylbenzoic кислота
- dimethyl p-octadecyl phenylsulfonyl amino propyl ammoium propylsulfonate
- Тозилхлорид
- Кислотно-желтый 79
- 2-Метил-4-нитроанилина
1of6
Бензолсульфонилхлорид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-13131129325 | China | 5887 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 |