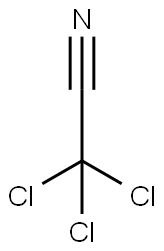
Трихлорацетонитрил
- английское имяTrichloroacetonitrile
- CAS №545-06-2
- CBNumberCB5853801
- ФормулаC2Cl3N
- мольный вес144.39
- EINECS208-885-7
- номер MDLMFCD00001842
- файл Mol545-06-2.mol
| Температура плавления | -42 °C |
| Температура кипения | 83-84 °C(lit.) |
| плотность | 1.44 g/mL at 25 °C(lit.) |
| давление пара | 58 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | None |
| температура хранения | Store below +30°C. |
| растворимость | <0.1 g/100 mL at 21.5°C |
| форма | Liquid |
| цвет | Clear colorless to very slightly yellow |
| Запах | odor of chloral and hydrogen cyanide |
| Растворимость в воде | <0.1 g/100 mL at 21.5 ºC |
| Чувствительный | Lachrymatory |
| Мерк | 14,9628 |
| БРН | 605572 |
| Пределы воздействия | NIOSH: IDLH 25 mg/m3 |
| Диэлектрическая постоянная | 4.6(60℃) |
| Стабильность | Stable, but water sensitive. Incompatible with acids, water, steam. May hydrolyze in alkali or acid conditions. Flammable. |
| Справочник по базе данных CAS | 545-06-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-2 |
| FDA UNII | 6397DL8869 |
| МАИР | 3 (Vol. 52, 71) 1999 |
| Справочник по химии NIST | Acetonitrile, trichloro-(545-06-2) |
| Система регистрации веществ EPA | Trichloroacetonitrile (545-06-2) |
| UNSPSC Code | 12352117 |
| NACRES | NA.22 |
| Коды опасности | T,N | |||||||||
| Заявления о рисках | 23/24/25-51/53 | |||||||||
| Заявления о безопасности | 45-61 | |||||||||
| РИДАДР | UN 3276 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | AM2450000 | |||||||||
| Примечание об опасности | Toxic/Lachrymatory | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29269095 | |||||||||
| Банк данных об опасных веществах | 545-06-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 0.25 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301+H311+H331:Токсично при проглатывании, при контакте с кожей или при вдыхании.
H411:Токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
Трихлорацетонитрил химические свойства, назначение, производство
Химические свойства
colourless to slightly yellow liquidИспользование
Trichloroacetonitrile is involved as a reagent in Overman rearrangement, which is used to prepare alylic amines from allylic alcohols. It is also used to prepare bistrichloroacetimidates from diols leading to dihyrooxazines through acid catalyzed cyclization. Further, it is utilized in the synthesis of trichloroacetimidates by 1,8-Diazobicyclo[5.4.0]undec-7-ene (DBU) catalyzed addition of allylic alcohols. It finds application in the study of the methoxy methyl (MOM) catalyzed aza-Claisen rearrangement.Методы производства
Trichloroacetonitrile can be obtained by dehydration of trichloroacetamide with phosphorous pentoxide or by chlorination of acetonitrile with chlorine. Vapor phase chlorination in the presence of water and photochemical chlorination in the presence of catalysts such as HgCl2 or AlCl3 have been reported. Trichloroacetonitrile is an organic intermediate used, for example, in the synthesis of the fungicide etridiazole.Реакции воздуха и воды
Highly flammable. Insoluble in water.Профиль реактивности
May be sensitive to light and heat. Trichloroacetonitrile may react with water, steam, acid or acid fumes. Trichloroacetonitrile may hydrolyze under acidic or alkaline conditions. . The reaction of benzene and Trichloroacetonitrile evolves toxic chloroform and HCl gasses. (Hagedorn, F., H.-P. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).Опасность
Strong irritant to tissue. Questionable carcinogen.Пожароопасность
Trichloroacetonitrile is combustible.Профиль безопасности
Poison by ingestion and intravenous routes. Moderately toxic by inhalation and skin contact. Human mutation data reported. A skin and severe eye irritant. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition or in reaction with water, steam, acid, or acid fumes it produces toxic fumes of CN-, Cl-, and NOx. Used as an insecticide. See also NITRILES and CYANIDE.Оценка токсичности
Trichloroacetonitrile (TCAN) is a by-product of the chlorine disinfection of water containing natural organic material. When administered by gavage to pregnant Long-Evans rats in a medium-chain triglyceride vehicle, tricaprylin oil (Tricap), at a volume of 10 ml/kg, TCAN induced fetal cardiovascular anomalies at doses as low as 1 mg/kg/d. Five groups of approximately 20 pregnant female rats received TCAN in CO at 15, 35, 55, and 75 mg/kg/d and in Tricap at 15 mg/kg/d (10 ml/kg closing volume). Corn oil, Tricap, and water served as vehicle controls. Animals were treated by oral intubation on gestation d 6-18 (vaginal plug -= d 0). Five out of 20 dams (75 mg/kg) died during treatment. Adjusted maternal weight gain was lower in females receiving 35 mg/kg TCAN or greater. The mean percent of nonlive implants per litter was elevated at 55 and 75 mg/kg TCAN (CO). The TCAN dose-response curve for fetal (but not maternal)effects was shifted to the right when CO was compared to Tricap. Fetal weight was reduced at 15 mg/kg TCAN (Tricap) and at 55 mg/kg TCAN (CO). When TCAN was administered in CO, the mean frequency of soft-tissue maliformations decreased with significantly fewer septal and great vessel cardiovascular defects observed. The lowest observed adverse effect level for TCAN (CO) was determined to be 35 kg/kg.Трихлорацетонитрил запасные части и сырье
Трихлорацетонитрил поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-852-30606658 | China | 43340 | 58 | ||
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |
Трихлорацетонитрил Обзор)
1of4