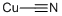
Медь (I) цианида
- английское имяCopper(I) Cyanide
- CAS №544-92-3
- CBNumberCB0193966
- ФормулаCCuN
- мольный вес89.56
- EINECS208-883-6
- номер MDLMFCD00010975
- файл Mol544-92-3.mol
| Температура плавления | 474 °C(lit.) |
| Температура кипения | decomposes [STR93] |
| плотность | 2.92 g/mL at 25 °C(lit.) |
| Плотность накопления | 750kg/m3 |
| давление пара | 0Pa at 25℃ |
| температура хранения | Poison room |
| растворимость | insoluble in H2O, ethanol; soluble in KCN solution |
| форма | Powder |
| Удельный вес | 2.92 |
| цвет | White-beige to greenish |
| Растворимость в воде | Practically insoluble in water and alcohol. Soluble in ammonium hydroxide, aqueous ammonia, pyridine and N-methylpyrrolidone. |
| Чувствительный | air sensitive |
| Мерк | 14,2661 |
| БРН | 3587244 |
| Константа произведения растворимости (Ksp) | pKsp: 19.46 |
| Пределы воздействия | TLV-TWA 1 mg Cu/m3 (ACGIH). |
| Стабильность | Stable. Incompatible with acids, bases, magnesium. Reacts violently with oxidizing agents, nitrates. Reaction with acid releases highly toxic gas (HCN). |
| LogP | -1.49 at 25℃ |
| Справочник по базе данных CAS | 544-92-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 534K22856J |
| Система регистрации веществ EPA | Copper(I) cyanide (544-92-3) |
| UNSPSC Code | 12352302 |
| NACRES | NA.22 |
| Коды опасности | T+,N,T | |||||||||
| Заявления о рисках | 26/27/28-32-50/53 | |||||||||
| Заявления о безопасности | 7-28-29-45-60-61-28A | |||||||||
| РИДАДР | UN 1587 6.1/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | GL7150000 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2837 19 00 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 544-92-3(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H300+H310+H330:Смертельно при проглатывании, при контакте с кожей или при вдыхании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P262:Избегать попадания в глаза, на кожу или одежду.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352+P310:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Немедленно обратиться за медицинской помощью.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
Медь (I) цианида химические свойства, назначение, производство
Описание
off-white to green powder. Insoluble in water,soluble in HCI, Nl40H, and potassium cyanide. Used in Sandmeyer's reaction to synthesize aryl cyanides. Toxic by skin absorption, through open wounds, by ingestion, and by inhalation of hydrogen cyanide that arises from slight decomposition. Produces toxic oxides of nitrogen in fires.Химические свойства
Cuprous cyanide is a white crystalline substance.Физические свойства
Cream-colored powder or green orthorhombic or red monoclinic crystals; density 2.90 g/cm3; melts at 474°C; decomposes at higher temperatures; practically insoluble in water, ethanol, and cold dilute acids; dissolves in ammonium hydroxide and potassium cyanide solutions.Использование
Copper(I) cyanide is commonly used as a polymerization catalyst. The compound is useful in organic synthesis, as a catalyst, reagent in the preparation of nitriles(sandmeyer reaction). It is also used in electroplating copper and iron, silver plating, brass plating, and copper-tin alloy plating. It is used to make phthalocyanine dyes and pigments.Профиль реактивности
Copper(I) Cyanide is decomposed by acids to give off hydrogen cyanide, a flammable poisonous gas. Tends to explosive instability. Capable of violent oxidation under certain condition: fusion with metal chlorates, perchlorates, nitrates or nitrites can cause explosions [Bretherick, 1979 p. 101]. Reacts with incandescence with magnesium [Mellor, 1940, Vol. 4, 271].Опасность
Poison.Угроза здоровью
Cuprous cyanide is a highly toxic substance. The toxic routes are inhalation of dust, ingestion, and skin contact. Toxicology and LD50 values for this compound are not reported. Because it is slightly soluble in water, its dissociation to cuprous and cyanide ions in the body may not be significant. The role of cyanide ion in the toxicity of cuprous cyanide is not established. The inhalation hazard, however, is attributable to copper. It is a skin irritant.Пожароопасность
Special Hazards of Combustion Products: Toxic hydrogen cyanide gas may form in fires.Профиль безопасности
A poison. Reacts violently with magnesium. When heated to decomposition it emits very toxic CNand NOx. See also CYANIDE and COPPER COMPOUNDS.Возможный контакт
Copper cyanide is used in electroplating copper on iron; and as an insecticide and a catalyst.Перевозки
UN1587 Copper cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materialsМетоды очистки
Wash the cyanide thoroughly with boiling H2O, then with EtOH. Dry it at 100o to a fine soft powder. It dissolves in excess alkali cyanide solutions to form the very soluble complex ion Cu(CN)43-. [Bassett & Corbett J Chem Soc 125 1660 1924, Barber J Chem Soc 79 1943.]Несовместимости
Contact with heat, strong acids (HCl, H2SO4, HNO3) forms deadly hydrogen cyanide gas. May release hydrogen cyanide on contact with moisture. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acetylene gas, and chemically active metals, such as potassium, sodium, magnesium, and zinc.Утилизация отходов
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill. Copper-containing wastes can be concentrated to the point where copper can be electrolytically removed and reclaimed. If recovery is not feasible, the copper can be precipitated by alkali; the cyanide destroyed by alkaline oxidation yielding a sludge which can be sent to a chemical waste landfill. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.Медь (I) цианида запасные части и сырье
сырьё
1of3
запасной предмет
- 1H-ИНДАЗОЛ-5-КАРБОНИТРИЛ
- 2-Амино-4-метилбензонитрил
- Пиразинкарбoнитрил
- Benzoyl cyanide
- 6-(AMINOMETHYL)-3-AMINOPYRIDINE
- 5-бромпиридин-2-карбоновой кислоты
- ПИРУВОНИТРИЛ
- 8-CHLORO-2-(TRIFLUOROMETHYL)QUINOLINE-4-CARBONITRILE
- 3-Cyanoquinoline
- 2-Cyano-5-methylpyridine
- 2,5-DIMETHYLBENZONITRILE
- 4-бром-2-фторбензонитрил
- C- (1H-ИНДОЛ-5-YL) -МЕТИЛАМИН
- 5-цианоиндол
- Disperse Blue 366
- МЕТИЛ-5-БРОМПИРАЗИН-2-КАРБОКСИЛАТ
- 1-бензотиофен-3-карбоновая кислота
- 3-Amino-2-pyridinecarbonitrile
- 2-циано-6-метилпиридин
- 6-METHOXYBENZOTHIAZOLE-2-CARBOXYLIC КИСЛОТА
- 6-AMINO-5-METHYLNICOTINONITRILE
- 3-Butenenitrile
- 5-ацетилтиофен-2-карбoнитрил
- 3,5-ДИФТОРО-БЕНЗАМИДИН ГИДРОХЛОРИД
- 5-(AMINOMETHYL)PYRIDIN-2-AMINE
1of8
Медь (I) цианида поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +8615866703830 | China | 8497 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |