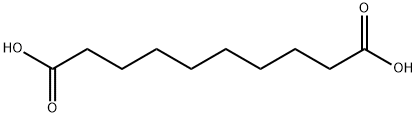
Себациновая кислота
- английское имяSebacic acid
- CAS №111-20-6
- CBNumberCB0329394
- ФормулаC10H18O4
- мольный вес202.25
- EINECS203-845-5
- номер MDLMFCD00004440
- файл Mol111-20-6.mol
| Температура плавления | 133-137 °C (lit.) |
| Температура кипения | 294.5 °C/100 mmHg (lit.) |
| Плотность накопления | 600-620kg/m3 |
| плотность | 1.21 |
| давление пара | 1 mm Hg ( 183 °C) |
| показатель преломления | 1.422 |
| Fp | 220 °C |
| температура хранения | Store below +30°C. |
| растворимость | ethanol: 100 mg/mL |
| форма | Powder or Granules |
| пка | 4.59, 5.59(at 25℃) |
| цвет | White to off-white |
| Запах | monoclinic prismatic tablets, wh. powd., fatty acid odor |
| Растворимость в воде | 1 g/L (20 ºC) |
| Мерк | 14,8415 |
| БРН | 1210591 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, bases, reducing agents. |
| ИнЧИКей | CXMXRPHRNRROMY-UHFFFAOYSA-N |
| LogP | 1.5 at 23℃ |
| Справочник по базе данных CAS | 111-20-6(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | SEBACIC ACID |
| FDA 21 CFR | 175.105; 175.300; 175.320 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 97AN39ICTC |
| Справочник по химии NIST | Decanedioic acid(111-20-6) |
| Система регистрации веществ EPA | Sebacic acid (111-20-6) |
| UNSPSC Code | 12352106 |
| NACRES | NA.22 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36-24/25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | VS0875000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29171310 | |||||||||
| Токсичность | LD50 orally in Rabbit: 3400 - 14500 mg/kg LD50 dermal Rat > 2000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P405:Хранить в недоступном для посторонних месте.
Себациновая кислота химические свойства, назначение, производство
Описание
Sebacic acid is a dicarboxylic acid with structure (HOOC)(CH2)8(COOH), and is naturally occurring.In its pure state it is a white flake or powdered crystal. The product is described as non-hazardous, though in its powdered form it can be prone to flash ignition (a typical risk in handling fine organic powders).
Sebaceus is Latin for tallow candle, sebum (tallow) is Latin for tallow, and refers to its use in the manufacture of candles. Sebacic acid is a derivative of castor oil, with the vast majority of world production occurring in China which annually exports over 20,000 metric tonnes, representing over 90 % of global trade of the product.
In the industrial setting, sebacic acid and its homologues such as azelaic acid can be used in plasticizers, lubricants, hydraulic fluids, cosmetics, candles, etc. Sebacic acid is also used as an intermediate for aromatics, antiseptics, and painting materials.
Химические свойства
White flaky crystals. Slightly soluble in water, soluble in alcohol and ether.Использование
Decanedioic acid was named by Thenard LJ (1802) from the Latin sebaceus(tallow candle) or sebum (tallow) in reference to its use in the manufacture of candles. Thenard LJ isolated this compound from distillation products of beef tallow. In 1954, it was reported that it was produced in excess of 10,000 tons annually by alkali fission of castor oil. Sebacic acid and its derivatives, as azelaic acid, have a variety of industrial uses as plasticizers, lubricants, diffusion pump oils, cosmetics, candles, etc. It is also used in the synthesis of polyamide, as nylon, and of alkyd resins. An isomer, isosebacic acid, has several applications in the manufacture of vinyl resin plasticizers, extrusion plastics, adhesives, ester lubricants, polyesters, polyurethane resins and synthetic rubber.Определение
ChEBI: Sebacic acid is an alpha,omega-dicarboxylic acid that is the 1,8-dicarboxy derivative of octane. It has a role as a human metabolite and a plant metabolite. It is an alpha,omega-dicarboxylic acid and a dicarboxylic fatty acid. It is a conjugate acid of a sebacate(2-) and a sebacate. It derives from a hydride of a decane.Подготовка
Sebacic acid is normally made from castor oil, which is essentially glycerol triricinoleate. The castor oil is heated with sodium hydroxide at about 250??e. This treatment results in saponification of the castor oil to ricinoleic acid which is then cleaved to give 2-octanol and sebacic acid: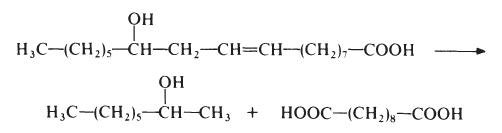
This process results in low yields of sebacic acid (about 50% based on the castor oil) but, nevertheless, other routes have not proved competitive. Sebacic acid is a colourless crystalline solid, m.p. 134??.
Общее описание
White granular powder. Melting point 153°F. Slightly soluble in water. Sublimes slowly at 750 mm Hg when heated to melting point.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Sebacic acid reacts exothermically to neutralize bases, both organic and inorganic. May react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Can react with active metals to form gaseous hydrogen and a metal salt. Such reactions are slow in the dry, but systems may absorb enough water from the air to allow corrosion of iron, steel, and aluminum parts and containers. Reacts slowly with cyanide salts to generate gaseous hydrogen cyanide. Reacts with solutions of cyanides to cause the release of gaseous hydrogen cyanide. May generate flammable and/or toxic gases and heat with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. May react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Can be oxidized exothermically by strong oxidizing agents and reduced by strong reducing agents. May initiate polymerization reactions.Пожароопасность
Flash point data for Sebacic acid are not available. Sebacic acid is probably combustible.Методы очистки
Purify sebacic acid via the disodium salt which, after crystallisation from boiling water (charcoal), is again converted to the free acid. The free acid is crystallised repeatedly from hot distilled water or from Me2CO/pet ether and dried under vacuum. [Beilstein 2 IV 2078.]Себациновая кислота запасные части и сырье
сырьё
1of3
запасной предмет
1of4
Себациновая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | ||
| +86-2102300 +86-18632882519 |
China | 300 | 58 | ||
| +8617653113209 | China | 3049 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +8613343047651 | China | 3692 | 58 |