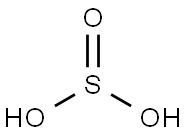
Сернистая кислота
- английское имяSulfurous Acid
- CAS №7782-99-2
- CBNumberCB6160852
- ФормулаH2O3S
- мольный вес82.08
- EINECS231-973-1
- номер MDLMFCD00011335
- файл Mol7782-99-2.mol
| плотность | 1.03 g/mL at 25 °C(lit.) |
| плотность пара | 2.3 (vs air) |
| растворимость | solution of SO2 in H2O |
| пка | 1.92(at 25℃) |
| форма | Liquid |
| цвет | Colorless |
| РН | pKa1= 1.658, pKa2 = 6.820(25℃) |
| Растворимость в воде | Miscible with water. |
| Чувствительный | Air Sensitive |
| Мерк | 14,8976 |
| Диэлектрическая постоянная | 15.6(0℃) |
| Стабильность | Stable. Incompatible with strong bases. |
| ИнЧИКей | LSNNMFCWUKXFEE-UHFFFAOYSA-N |
| LogP | -1.579 (est) |
| Вещества, добавляемые в пищу (ранее EAFUS) | SULFUROUS ACID |
| Справочник по базе данных CAS | 7782-99-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | J1P7893F4J |
| Система регистрации веществ EPA | Sulfurous acid (7782-99-2) |
| UNSPSC Code | 12352300 |
| NACRES | NB.24 |
| Коды опасности | C,Xn | |||||||||
| Заявления о рисках | 20-34-36/38 | |||||||||
| Заявления о безопасности | 26-36/37/39-45 | |||||||||
| РИДАДР | UN 1833 8/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | WT2775000 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28111990 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Сернистая кислота химические свойства, назначение, производство
Описание
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. However, there is no evidence that sulfurous acid exists in solution, while the molecules of which has been detected in the gas phase, since the reaction is reversible and the acid readily decomposes back into the reactants. Sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts.Sulfurous acid and its salts are commonly applied as powerful reducing agents and disinfectant agents due to its strong reducibility. It is also considered as a mild bleaching agent especially for applications having chlorine sensitive materials. Besides, Sulfurous acid is a food additive listed on the EAFUS food Additive Database (Jan. 2001).
Химические свойства
SULFUROUS ACID, colorless liquid, prepared by dissolving SO2 in H2O. Reagent grade H2SO3 contains approximately 6% SO2 in solutionИспользование
Sulfurous acid (H2SO3) can be produced by burning sulfur to form sulfur dioxide (SO2) gas and by then dissolving the gas in water to form sulfurous acid. This is the acid produced by burning coal that has a high sulfur content; the gaseous sulfur dioxide by-product of combustion then combines with atmospheric water to form “acid rain.”Определение
A weak acid found only in solution, made by passing sulfur(IV) oxide into water. The solution is unstable and smells of sulfur(IV) oxide. It is a reducing agent, converting iron(III) ions to iron(II) ions, chlorine to chloride ions, and orange dichromate(VI) ions to green chromium(III) ions.Общее описание
Sulfurous acid, H2S03, is an unstable, water-soluble, colorless liquid with a pungent burning sulfur odor. Corrosive to metals and tissue. It is derived from absorption of sulfur dioxide( S02) in water.Reagent grade sulfurous acid contains about 6% sulfur dioxide in solution. Sulfurous acid is a strong reducing agent.It is readily oxidized to sulfuric acid and is itself reduced to hydrogen sulfide by zinc and dilute sulfuric acid. Sulfurous acid is used in the synthesis of medicines and chemicals, in the manufacture of paper and wine, in brewing, in metallurgy, in ore flotation, as a bleach and analytical reagent, and for refining of petroleum products.Реакции воздуха и воды
Soluble in water with release of heat.Профиль реактивности
Colorless, corrosive, moderately toxic liquid. When heated to decomposition SULFUROUS ACID emits toxic fumes of oxides of sulfur [Lewis, 3rd ed., 1993, p. 1196].Опасность
Toxic by ingestion and inhalation, strong irritant to tissue.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Промышленное использование
Sulfurous acid (H2SO3) is usually marketed as liquid SO2. The bulk of SO2 is produced from off-smelter gases. Although handling of SO2 liquid requires special equipment, it is frequently used as a pH regulator and depressant, primarily during the treatment of complex sulfide ores. SO2 is largely used in North American operations as a pyrite depressant and for the depression of galena during copper/lead separation.Профиль безопасности
A poison by ingestion and inhalation. A corrosive irritant to skin, eyes, and mucous membranes. Human systemic effects by ingestion: nausea or vomiting, hypermotility, diarrhea, and othergastrointestinal effects. When heated to decomposition it emits highly toxic fumes ofSOx.использованная литература
https://en.wikipedia.org/wiki/Sulfurous_acidhttp://printwiki.org/Sulfurous_Acid
http://www.softschools.com/formulas/chemistry/sulfurous_acid_uses_properties_structure_formula/242/
https://pubchem.ncbi.nlm.nih.gov/compound/Sulfurous_acid#section=Top
http://www.chemistrylearner.com/sulfurous-acid.html
Сернистая кислота запасные части и сырье
запасной предмет
- whitener WG for wool
- sulfonated ligno-sulfomethylated phenolic resin copolymer
- 3-Indazolinone
- Таурин
- hydrogen [4-[[4-(diethylamino)phenyl][4-[ethyl[(3-sulphonatobenzyl)amino]-o-tolyl]methylene]-3-methylcyclohexa-2,5-dien-1-ylidene](ethyl)(3-sulphonatobenzyl)ammonium, sodium salt
- 3,7-диметил-7-гидроксиоктанал
- Direct Black 168
- DL-триптофан
- DIRECT VIOLET 51
- (4S)-HYDROXY-3-METHYL-2-(2-PROPENYL)-2-CYCLOPENTENE-1-ONE
1of8
Сернистая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-0571-87191913 +86-19957014543 |
CHINA | 144 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +8617731987558 | China | 989 | 58 | |
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | |
| +8617732866630 | China | 18147 | 58 |