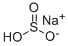
Натрия бисульфид
- английское имяSodium bisulfite
- CAS №7631-90-5
- CBNumberCB5854257
- ФормулаHNaO3S
- мольный вес104.06
- EINECS231-548-0
- номер MDLMFCD00003530
- файл Mol7631-90-5.mol
химическое свойство
| Температура плавления | 150 °C |
| плотность | 1.48 |
| давление пара | 40 hPa ( 20 °C) |
| температура хранения | Store at RT. |
| растворимость | 300 g/L |
| форма | Powder/Solid |
| Удельный вес | 1.48 |
| цвет | White |
| РН | 4.5 (20°C in H2O) |
| Запах | Slight odor of sulfur dioxide |
| Растворимость в воде | 300 g/L |
| Мерк | 13,8660 |
| Стабильность | Stable. Incompatible with strong oxidizing agents, strong acids. |
| ИнЧИКей | DWAQJAXMDSEUJJ-UHFFFAOYSA-M |
| FDA 21 CFR | 182.3739; 582.3739; 161.173 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SODIUM BISULFITE |
| Специальный комитет по веществам GRAS | Sodium bisulfite |
| Справочник по базе данных CAS | 7631-90-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2-3 |
| FDA UNII | TZX5469Z6I |
| Система регистрации веществ EPA | Sodium bisulfite (7631-90-5) |
| UNSPSC Code | 12352302 |
| NACRES | NA.22 |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-31-41-52 | |||||||||
| Заявления о безопасности | 26-39-46-25 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3 | |||||||||
| РИДАДР | UN 2693 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | UX8225000 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28321000 | |||||||||
| Банк данных об опасных веществах | 7631-90-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 i.v. in rats: 115 mg/kg (Hoppe, Goble) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Натрия бисульфид химические свойства, назначение, производство
Химические свойства
Sodium bisulfite is a white crystalline solid. Slight odor of sulfur dioxide and a disagreeable taste. Slowly oxidized to the sulfate on exposure to airИспользование
sodium bisulfite is a preservative and anti-oxidant, it is most frequently used as a pH adjuster.Общее описание
Also known as sodium acid sulfite or sodium hydrogen sulfite, NaHS03 is white crystals or crystalline powder that has a slight sulfurous odor and taste.Specific gravity 1.48. Strong irritant to skin and tissue. It is soluble in water,insoluble in alcohol,unstable in air,and non-combustible. Derived by saturating sodium carbonate solution with sulfur dioxide and the solution crystallized. Used as a chemical(sodium salts, cream of tartar), in vat dye preparation, textiles (antichlor, mordant, discharge), food preservative, bleaching groundwood, wool,etc., reducing agent, fermentation, antiseptic, cask sterilization (brewing), copper and brass plating,color preservative for pale crepe rubber, wood pulp digestion, analytical reagent,and as a dietary supplement.Реакции воздуха и воды
Water soluble.Профиль реактивности
Sodium bisulfite is a reducing agent. Emits highly toxic gaseous sulfur dioxide gas when heated to decomposition or on contact with mineral acids [Sax, 9th ed., 1996, p. 2949].Опасность
Not permitted in meats and other sources of vitamin B 1 , strong irritant to skin and tissue.Угроза здоровью
Powder is irritating to eyes, nose, and throat and can irritate skin. Ingestion may cause irritation of stomach. Very large doses cause violent colic, diarrhea, depression, and death.Пожароопасность
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.Контактные аллергены
Sodium bisulfite is mainly used as an antioxidant in pharmaceutical products, as a disinfectant or bleach, and in the dye industry. The bisulfite of commerce consists chiefly of metabisulfite and possesses the same properties as the true bisulfite. So, the allergen to be tested in products containing disulfite is the corresponding metabisulfite.Профиль безопасности
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. Mutation data reported. An allergen. When heated to decomposition it emits toxic fumes of SO, and Na2O. See also SULFUROUS ACID and SULFITESВозможный контакт
Sodium bisulfite is used in the digestion of wood pulp, in the tanning of leather; in the dyeing of textiles; as a photographic reducing agent; as a food preservative; as an additive in electroplating; as disinfectant, bleach, antioxidant, and as inhibitor of yeast and bacteria in winemaking.Перевозки
UN2693 Bisulfites, inorganic, aqueous solutions, n.o.s., Hazard class: 8; Labels: 8-Corrosive material. UN3260 Bisulfites, inorganic, solid, n.o.s., Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Crystallise it from hot H2O (1mL/g). Dry it at 100o under vacuum for 4hours.Несовместимости
Aqueous solution is a weak acid. Incompatible with strong mineral acids forming a toxic sulfur dioxide gas. A strong reducing agent, sodium bisulfite is incompatible with oxidizers, such as perchlorates, peroxides, permanganates, chlorates, and nitrates. Reacts with bases forming sulfate. Slowly oxidizes to sulfate in air. Heat causes decomposition. Slowly oxidized to the sulfate on exposure to air. Contact with oxidizers or acids forms sulfur dioxide gas. Attacks some metals in the presence of moisture.Утилизация отходов
Dump into water, add soda ash, then neutralize with HCl; flush to sewer with large volumes of water.Натрия бисульфид запасные части и сырье
сырьё
1of2
запасной предмет
- 2,6-дибромфенола
- N,N-Diethylcyanoacetamide
- N,N'-Дифенилбензидин
- 4-ацетилбензойная кислота
- анамицина сульфа
- 3-Bromoindazole
- 1-Hydroxy-3-phenyl-1,3-propanedisulfonic acid disodium salt
- 2,4-гидрат Dinitrobenzenesulfonic кислота
- Бенз [cd] индол-2 (1H) -он
- 3-Aminobenzaldehyde
- FUBERIDAZOLE
- 2,3-дигидро-бензо [Ь] фуран-5-бороновой кислоты
- N-(1-нафтил) этилендиамина дигидрохлорид
- 4-йоданилин
- 5-гидроксихинолин
- 2-Methoxyphenylacetone
- 1-Cyclopentenecarbonitrile
- 4'-метокси-2'-nitroacetanilide
- Быстрый синий BB
- Реактивный синий 104
- PIPERITONE
- 3,3-Dimethylacrylic кислота
- Dichlorosulfophenyl-3-methylpyrazolone
- 3,3'-Dihydroxybenzidine
- 2-[(4-аминофенил)сульфонил]этилгидросульфат
1of8
Натрия бисульфид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 |
Натрия бисульфид Обзор)
1of4