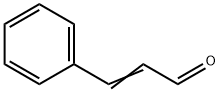
Cinnamaldehyde
- английское имяCinnamaldehyde
- CAS №104-55-2
- CBNumberCB1168459
- ФормулаC9H8O
- мольный вес132.16
- EINECS203-213-9
- номер MDLMFCD00007000
- файл Mol104-55-2.mol
| Температура плавления | −9-−4 °C(lit.) |
| Температура кипения | 248 °C (lit.) |
| плотность | 1.05 g/mL at 25 °C (lit.) |
| плотность пара | 4.6 (vs air) |
| давление пара | <0.1 hPa (20 °C) |
| FEMA | 2286 | CINNAMALDEHYDE |
| показатель преломления | n |
| Fp | 160 °F |
| температура хранения | Store below +30°C. |
| растворимость | 1g/l soluble |
| форма | Liquid |
| пка | 0[at 20 ℃] |
| Удельный вес | 1.05 |
| цвет | Clear yellow |
| Запах | Strong odor of cinnamon |
| Odor Type | spicy |
| Биологические источники | cassia oil |
| Вязкость | 21.25mm2/s |
| Растворимость в воде | Slightly soluble |
| Мерк | 13,2319 |
| Номер JECFA | 656 |
| Диэлектрическая постоянная | 16.9(24℃) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| ИнЧИКей | KJPRLNWUNMBNBZ-QPJJXVBHSA-N |
| LogP | 1.83-2.1 at 25-27℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | CINNAMALDEHYDE |
| FDA 21 CFR | 182.60 |
| Справочник по базе данных CAS | 104-55-2(CAS DataBase Reference) |
| FDA UNII | SR60A3XG0F |
| Справочник по химии NIST | Cinnamylaldehyde(104-55-2) |
| Система регистрации веществ EPA | Cinnamaldehyde (104-55-2) |
| UNSPSC Code | 12352114 |
| NACRES | NA.22 |
| Коды опасности | Xi |
| Заявления о рисках | 36/37/38-43 |
| Заявления о безопасности | 26-36/37 |
| РИДАДР | UN8027 |
| WGK Германия | 3 |
| RTECS | GD6476000 |
| F | 10-23 |
| кода HS | 29122900 |
| Банк данных об опасных веществах | 104-55-2(Hazardous Substances Data) |
| Токсичность | LD50 in rats (mg/kg): 2220 orally (Jenner) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Cinnamaldehyde химические свойства, назначение, производство
Описание
Cinnamic aldehyde is used as a flavoring agent, ingredient of fragrance in soft drinks, ice creams, dentifrices, pastries, chewing-gum, etc. It can induce both contact urticaria and delayed-type reactions. It can be implicated in contact dermatitis in those who work in the perfume industry or food handlers. Cinnamic aldehyde is contained in the "fragrance mix".Химические свойства
Cinnamaldehyde exists as yellowish to greenish-yellow oily liquid with a Strong pungent, spicy, cinnamon odor. It is normally insoluble in water and many organic solvents but is miscible with alcohol and other flavoring oils. Exposure to air will result in thickening and oxidation.
Cinnamaldehyde (cinnamic aldehyde, benzylideneactaldehyde, phenylacrolein, 3-phenylpropenal, 3-phenyl-2-propenal) is a saturated aldehyde with an aromatic ring that is a yellowish, oily liquid at room temperature. It is used as a flavoring and aromatic agent in a variety of food products, perfumes, and household products. It has also seen use as a rubber reinforcing agent. A burning taste that produces the odor and flavor of the spice may be found with this aromatic aldehyde. Cinnamaldehyde has also been used as an attractant for insect control, in the preparation of corrosion inhibitors, and as a coating for metals. Although extensively used in industry, it is also a natural constituent of cinnamon leaves and bark, some essential oils, and other plant products.
Вхождение
Reported found in celery seed, cinnamon, cinnamon leaf, cassia leaf, clove stem and lemon balm.Использование
Cinnamaldehyde is used in flavor and perfumes.It occurs in cinnamon oils.Общее описание
Yellow oily liquid with a cinnamon odor and sweet taste.Реакции воздуха и воды
Thickens on exposure to air. May be unstable to prolonged exposure to air. Slightly water soluble .Профиль реактивности
Cinnamaldehyde reacts with sodium hydroxide owing to aerobic oxidation.Угроза здоровью
Cinnamaldehyde can cause moderate to severeskin irritation. Exposure to 40 mg in48 hours produced a severe irritation effecton human skin. The toxicity of this compoundwas low to moderate on test subjects,depending on the species and the toxicroutes. However, when given by oral routein large amounts, its poisoning effect wassevere. Amounts greater than 1500 mg/kghave produced a wide range of toxic effectsin rats, mice, and guinea pigs. The symptomswere respiratory stimulation, somnolence,convulsion, ataxia, coma, hypermotility, anddiarrhea.LD50 value, oral (guinea pigs): 1150 mg/kg
Cinnamaldehyde is a mutagen. Its carcinogeniceffect is not established.
Пожароопасность
Cinnamaldehyde is combustible.Сельскохозяйственное использование
Fungicide, Insecticide: Used as an antifungal agent, corn rootworm attractant, and dog and cat repellent. Can be used on soil casing for mushrooms, row crops, turf and all food commodities. Not listed for use in EU countries.Торговое название
ADIOS®; ZIMTALDEHYDE®; ZIMTALDEHYDE® LIGHTКонтактные аллергены
This perfumed molecule is used as a fragrance in perfumes, a flavoring agent in soft drinks, ice creams, dentifrices, pastries, chewing-gum, etc. It can induce both contact urticaria and delayed-type reactions. It can be responsible for dermatitis in the perfume industry or in food handlers. Cinnamic aldehyde is contained in “fragrance mix.” As a fragrance allergen, it has to be mentioned by name in cosmetics within the EU.разработк И противоопухолевых препаратов
This is promising in antitumor activity against NSCLC cells. The cells were inducedin apoptosis and also the epithelial-mesenchymal transition was reversed affectingthe Wnt/b-catenin pathway (Bouyahya et al. 2016).Профиль безопасности
Poison by intravenous and parenteral routes. Moderately toxic by ingestion and intraperitoneal routes. A severe human skin irritant. Mutation data reported. Combustible liquid. May ipte after a delay period in contact with NaOH. When heated to decomposition it emits acrid smoke and fumes. See also ALDEHYDES.Возможный контакт
Botanical fungicide and insecticide. Used as an antifungal agent, corn rootworm attractant, and dog and cat repellent. Can be used on soil casing for mushrooms, row crops, turf, and all food commodities. Not listed for use in EU countries.Перевозки
UN1989 Aldehydes, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquidНесовместимости
Aldehydes are frequently involved in selfcondensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, ketones, azo dyes, caustics, boranes, hydrazinesУтилизация отходов
Incineration. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers.Cinnamaldehyde запасные части и сырье
Cinnamaldehyde поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | ||
| +86-13806087780 | China | 17365 | 58 | ||
| -- | China | 6567 | 58 | ||
| +8613393234347 | China | 2946 | 58 | ||
| +86-15662691337 +86-15662695772 |
China | 998 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 |
Cinnamaldehyde Обзор)
1of4