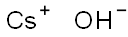
Гидроксид цезия
- английское имяCesium hydroxide
- CAS №21351-79-1
- CBNumberCB7357929
- ФормулаCsHO
- мольный вес149.91
- EINECS244-344-1
- номер MDLMFCD00149664
- файл Mol21351-79-1.mol
| Температура плавления | 272 °C(lit.) |
| плотность | 3.68 g/mL at 25 °C(lit.) |
| растворимость | Miscible with ethanol. |
| форма | Liquid |
| пка | 11.6[at 20 ℃] |
| цвет | Colorless |
| Растворимость в воде | soluble |
| Чувствительный | Air Sensitive |
| Мерк | 13,2022 |
| Пределы воздействия | ACGIH: TWA 2 mg/m3 NIOSH: TWA 2 mg/m3 |
| Стабильность | Stable. Incompatible with acids. Absorbs carbon dioxide from the air. |
| Справочник по базе данных CAS | 21351-79-1(CAS DataBase Reference) |
| FDA UNII | 458ZFZ6235 |
| Справочник по химии NIST | Cesium hydroxide(21351-79-1) |
| Система регистрации веществ EPA | Cesium hydroxide (21351-79-1) |
| UNSPSC Code | 12352302 |
| NACRES | NA.23 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 22-35-34 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-37/39-27-28 | |||||||||
| РИДАДР | UN 2682 8/PG 2 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 2 mg/m3 | |||||||||
| WGK Германия | 3 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28259090 | |||||||||
| Банк данных об опасных веществах | 21351-79-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 i.p. in rats: 100 mg/kg (Cochran) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
Гидроксид цезия химические свойства, назначение, производство
Химические свойства
Cesium hydroxide is a colorless-to-yellow crystalline compound. It is often used in a water solution.Физические свойства
White to yellowish fused crystalline mass; highly deliquescent; very alkaline; density 3.68 g/cm3; melts 272°C; highly soluble in water; soluble in ethanol; aqueous solution is very alkaline.Использование
Cesium hydroxide is used in the preparation of porous cesium titanosilicates, which are potential ion-exchange materials utilized for radioactive wastes cleaning purpose. It is also involved in the preparation of other cesium salts. Further, it is used as an electrolyte in alkaline storage batteries and as a catalyst in organic synthesis. In addition to this, it is used in color photography.прикладной
Cesium hydroxide also exerts unusual effects in organic reactions, such as the controlled alkylation of amines. In this instance, the cesium ion itself is explicitly involved in the reaction. The hydroxide base promotes an alkylation of a primary amine, while the Cs+ ion weakly coordinates to the amine such that further alkylation is inhibited, preventing formation of dialkyl and trialkyl amines.Cesium hydroxide solution is used in industrial catalysis. Cesium acts as apromoter e.g. in the production of polyols. Polyols are important raw materials for the production of polyurethane foams. Their synthesis profits from use of cesium hydroxide as base catalyst. In polyol synthesis isomerization of the starting material propylene oxide is an adverse side reaction eventually leading to chain termination in the polymerization with polyisocyanate. The tendency of polyols to undergo isomerization is minimized when using cesium hydroxide, as isomerization tendency decreases in the order Li > Na > K > Rb > Cs.
Подготовка
Cesium hydroxide is prepared by electrolysis of cesium salts to obtain cesium metal, which then reacts with water to yield hydroxide. It also is prepared by the action of barium hydroxide with an aqueous solution of cesium sulfate.Определение
ChEBI: Caesium hydroxide is an alkali metal hydroxide and a caesium molecular entity.Общее описание
Cesium hydroxide is a colorless to yellow crystalline solid. Harmful to skin and eyes. Used in electric storage batteries.Реакции воздуха и воды
Dilution with water may generate enough heat to cause steaming or spattering.Профиль реактивности
CESIUM HYDROXIDE,SOLUTION neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].Опасность
A poison. Skin, eye, and upper respiratory tract irritant.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Профиль безопасности
Poison by intraperitoneal route. Moderately toxic by ingestion. A powerful caustic. A corrosive skin and eye irritant. See also CESIUM.Возможный контакт
Cesium hydroxide may be used as a raw material for other cesium salts; such as the chloride which in turn may be used to produce cesium metal. Cesium metal is used in electronic devices.Перевозки
UN2682 Cesium hydroxide, Hazard class: 8; Labels: 8-Corrosive material. UN2681 Cesium hydroxide, solution, Hazard class: 8; Labels: 8-Corrosive materialНесовместимости
Cesium hydroxide is the strongest base known. Keep away from all acids. It must be stored in silver or platinum and out of contact with air because of its reactivity with glass. CsOH causes the generation of considerable heat in contact with water or moisture. Contact with many organic compounds, many metals (i.e., aluminum, lead, tin, zinc), glass, oxygen, or carbon dioxide causes a violent reaction.Гидроксид цезия запасные части и сырье
Гидроксид цезия поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +8617013299288 | China | 12373 | 58 | |
| +86-852-30606658 | China | 24727 | 58 |