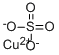
СУЛЬФАТ МЕДИ (II)
- английское имяCopper(II) sulfate
- CAS №7758-98-7
- CBNumberCB7751862
- ФормулаCuO4S
- мольный вес159.61
- EINECS231-847-6
- номер MDLMFCD00010981
- файл Mol7758-98-7.mol
| Температура плавления | 200 °C (dec.)(lit.) |
| плотность | 3.603 g/mL at 25 °C(lit.) |
| Плотность накопления | 800kg/m3 |
| давление пара | 7.3 mm Hg ( 25 °C) |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | H2O: soluble |
| форма | powder |
| цвет | Slightly greenish to gray |
| Удельный вес | 3.603 |
| РН | 3.5-4.5 (50g/l, H2O, 20℃) |
| Водородный показатель | 3.7 - 4.5 |
| Flame Color | Bluish-green |
| Запах | odorless |
| Растворимость в воде | 203 g/L (20 ºC) |
| Чувствительный | Hygroscopic |
| Мерк | 14,2653 |
| Диэлектрическая постоянная | 10.3(0.0℃) |
| Пределы воздействия | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Стабильность | hygroscopic |
| ИнЧИКей | ARUVKPQLZAKDPS-UHFFFAOYSA-L |
| Справочник по базе данных CAS | 7758-98-7(CAS DataBase Reference) |
| FDA 21 CFR | 184.1261; 310.545; 558.258; 558.95; 582.80 |
| Вещества, добавляемые в пищу (ранее EAFUS) | COPPER SULFATE |
| Специальный комитет по веществам GRAS | Copper (cupric) sulfate |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | KUW2Q3U1VV |
| Код УВД | V03AB20 |
| Справочник по химии NIST | Cupric sulfate(7758-98-7) |
| Система регистрации веществ EPA | Cupric sulfate (7758-98-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,N,Xi | |||||||||
| Заявления о рисках | 36/38-50/53-22-51/53-36/37/38 | |||||||||
| Заявления о безопасности | 24/25-36-60-61-22-26 | |||||||||
| РИДАДР | UN 3288 6.1/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | GL8800000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2833 25 00 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 7758-98-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 481 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
СУЛЬФАТ МЕДИ (II) химические свойства, назначение, производство
Химические свойства
Cupric sulfate, a bluish crystalline powder, also known as hydrocyanite and copper sulfate, vitriol, chalcanthite, and bluestone, is an azure blue material used in the It is used in the leather industry. It is prepared by the reaction of sulfuric acid and copper. It is also obtained as a by-product from copper refineries.Использование
Copper(II) sulfate may be employed for the following studies:- As a catalyst for the acetylation of alcohols and phenols under solvent-free conditions.
- To compose the electrolyte for the electrodeposition of Cu-Zn-Sn precursors, required for the preparation of Cu2ZnSnS4 (CZTS) thin films.
- As a Lewis acid catalyst for the dehydration of alcohols.5
Определение
A compound prepared as the hydrate by the action of dilute sulfuric acid on copper( II) oxide or copper(II) carbonate. On crystallization, blue triclinic crystals of the pentahydrate (blue vitriol, CuSO4.5H2O) are formed. Industrially copper(II) sulfate is prepared by passing air through a hot mixture of dilute sulfuric acid and scrap copper. The solution formed is recycled until the concentration of the copper(II) sulfate is sufficient. Copper(II) sulfate is readily soluble in water. The monohydrate (CuSO4.H2O) is formed at 100°C and the anhydrous salt at 250°C. Anhydrous copper( II) sulfate is white; it is extremely hygroscopic and turns blue on absorption of water. It decomposes on heating to give copper(II) oxide and sulfur(VI) oxide.Copper(II) sulfate is used as a wood preservative, a fungicide (in Bordeaux mixture), and in the dyeing and electroplating industries.
Общее описание
A white or off-white solid. Melting point 200°C with decomposition. Non-combustible.Реакции воздуха и воды
Soluble in water.Профиль реактивности
Anhydrous Cupric sulfate serves as a weak oxidizing agent. Causes hydroxylamine to ignite. Gains water readily. The hydrated salt is vigorously reduced by hydroxylamine [Mellor 8:292(1946-1947)]. Both forms are incompatible with finely powdered metals. Both are incompatible with magnesium, corrode steel and iron, may react with alkalis, phosphates, acetylene gas, hydrazine, or nitromethane, and may react with beta-naphthol, propylene glycol, sulphathiazole and triethanolamine if the pH exceeds 7 . Both act as acidic salts, corrode metals and irritate tissues.Опасность
Toxic; highly irritant.Угроза здоровью
INGESTION: copper sulfate may induce severe gastroenteric distress (vomiting, gastroenteric pain, and local corrosion and hemorrhages), prostration, anuria, hematuria, anemia, increase in white blood cells, icterus, coma, respiratory difficulties, and circulatory failure.Сельскохозяйственное использование
Fungicide, Algaecide, Bactericide, Herbicide, Molluscicide: Copper sulfate is a fungicide used to control bacterial and fungal diseases of fruit, vegetable, nut, and field crops. These diseases include mildew, leaf spots, blights, and apple scab. It is used as a protective fungicide (Bordeaux mixture) for leaf application and seed treatment. It is also used as an algaecide and herbicide, and to kill slugs and snails in irrigation and municipal water treatment systems. It has been used to control Dutch elm disease. It is available as a dust, wettable powder, or liquid concentrate. Used as a fungicide and algaecide, in veterinary medicine and others. Copper sulfate is also used todetect and to remove trace amounts of water from alcohols and organic compounds.Промышленное использование
Copper sulfate (CuSO4·5H2O) is widely used as an activator for sphalerite, pyrite, pyrrhotite and other sulfides during processing of base metal ores. During flotation of some silicate minerals, copper sulfate is used as depressant, e.g. zirconium.In manufacturing copper sulfate, sulfuric acid and scrap copper metal are used. The process is based on the oxidation of metal and dissolution with H2SO4 according to the following reaction:
4Cu + O2 = 2Cu2O Cu2O + H2SO4 = CuSO4 + H2O 2Cu2SO4 + 2H2SO4 + O2 = 4CuSO4 + 2H2O Usually, in mineral processing applications, copper sulfate is delivered in crystal form.
Торговое название
AGRITOX®; BASICOP®; BCS COPPER FUNGICIDE®; BSC FLOWABLE®[C]; COPSIN®; CP BASIC SULFATE®; CUPROFIX®; FUNGI-SPERSE II[C]; SULTRACOB®; TNCS® 53; TRIANGLE®Профиль безопасности
A human poison by ingestion. An experimental poison by ingestion, subcutaneous, parenteral, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: gastritis, Qarrhea, nausea or vomiting, damage to kidney tubules, and hemolysis. Questionable carcinogen with experimental tumorigenic data. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. Reacts violently with hydroxylamine, magnesium. See also COPPER COMPOUNDS and SULFATES. When heated to decomposition it emits toxic fumes of SOxВозможный контакт
Copper sulfate is used as intermediate and wood preservative; also used in production of copper compounds; to detect and to remove trace amounts of water from alcohols and organic compounds; as a fungicide and algicide; in veterinary medicine and others.хранилище
Workers should keep copper sulfate stored in a cool, dry area with suffi cient ventilation. It should be kept away from alkalis, magnesium, ammonia, acetylene, and sodium hypobromite.Перевозки
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Методы очистки
After adding 0.02g of KOH to a litre of nearly saturated aqueous solution of the sulfate, it is left for two weeks, then the precipitate is filtered on to a fibreglass filter with pore diameter of 5-15 microns. The filtrate is heated to 90o and allowed to evaporate until some CuSO4.5H2O crystallises out. The solution is then filtered hot and cooled rapidly to give crystals which are freed from mother liquor by filtering under suction [Geballe & Giauque J Am Chem Soc 74 3513 1952]. Alternatively crystallise the sulfate from water (0.6mL/g) between 100o and 0o. The pentahydrate is slowly efflorescent, losing 2H2O at 30o, two more H2O are lost at 110o and a white anhydrous powder (dessicant) is obtained on heating above 250o.Несовместимости
Aqueous solution is an acid. May form explosive materials on contact with acetylene and nitromethane. Incompatible with strong bases; hydroxylamine, magnesium; zirconium, sodium hypobromite, hydrazine.Утилизация отходов
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill Add soda ash to waste CuSO4 solution; let stand 24 hours. Decant and neutralize solution before flushing to sewer. Landfill sludge.Меры предосторожности
During handling and use of copper sulfate, students and occupational workers should wear safety glasses and should not breathe the material in powder form. Copper sulfate is an environmental pollutant and must be carefully incorporated when used in its varied applications. Workers should wear protective clothing, goggles, impermeable gloves, and rubber boots to avoid skin contactСУЛЬФАТ МЕДИ (II) запасные части и сырье
сырьё
запасной предмет
- polyamide conductive fiber
- Cuprous thiocyanate
- 5-FLUORO-2-HYDROXY-3-METHYLBENZALDEHYDE
- Direct Blend Blue
- 2-(2-CHLOROETHOXY)-BENZENESULFONAMIDE
- 3-фторфенол
- 2-циано-5-метилбензолсульфанил хлорид
- 4-Chlorobenzenesulfonic acid
- 2-бром-5-нитротиазол
- Reactive Blue BRF
- zinc plating additive DPE-1
- Фталид
- Этил 2-хлор-4-метил-1,3-тиазол-5-карбоксила
- Solvent Violet 8
- Процион Синий H-5R
- 4-нитрофенил фениловый эфир
- Sulphur Brown 4
- 1,2:3,4-ди-O-изопропилиден-D-галактопираноза
- 4-фтор-2-нитробензонитрила
- Меди (II) глюкона
- РЕАКТИВНЫЙ ФИОЛЕТОВЫЙ 5
- 3-NITRO-PYRIDINE-2-CARBOXYLIC ACID
- Disperse Red 86
- Rubber peptizer
- 3-Phenoxytoluene
- trisodium [5-acetamido-4-hydroxy-3-[[2-hydroxy-5-[[2-(sulphooxy)ethyl]sulphonyl]phenyl]azo]naphthalene-2,7-disulphonato(5-)]cuprate(3-)
- trisodium [2-[(4,6-dichloro-1,3,5-triazin-2-yl)amino]-5-hydroxy-6-[(2-hydroxy-5-sulphophenyl)azo]naphthalene-1,7-disulphonato(5-)]cuprate(3-)
- 5-хлор-2-метилбензолдиазоний
- Меди (II) гидроксид
- Reactive Black 31
- 5-CHLORO-2-NITROANISOLE
- 2-ЭТИЛГЕКСАНОАТ МЕДИ (II)
- 6-БРОМФТАЛАЗИН-1(2Н)-ОН
- tetrasodium [mu-[[7,7'-(carbonyldiimino)bis[4-hydroxy-3-[(2-hydroxy-5-sulphophenyl)azo]naphthalene-2-sulphonato]](8-)]]dicuprate(4-)
- tetraammonium [mu-[[5,5'-[carbonylbis[imino(2-hydroxy-5-sulpho-p-phenylene)azo]]bis[6-hydroxynaphthalene-2-sulphonato]](8-)]]dicuprate(4-)
1of8
СУЛЬФАТ МЕДИ (II) поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +16837774622 | China | 295 | 58 | |
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-571 87238903 | CHINA | 988 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 |
СУЛЬФАТ МЕДИ (II) Обзор)
1of4