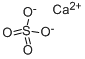
Кальция сульфат
- английское имяCALCIUM SULFATE
- CAS №7778-18-9
- CBNumberCB7151358
- ФормулаCa.O4S
- мольный вес136.14
- EINECS231-900-3
- номер MDLMFCD00799067
- файл Mol7778-18-9.mol
| Температура плавления | 1450 °C |
| плотность | 2.960 |
| показатель преломления | 1.575 |
| температура хранения | Store at RT. |
| растворимость | 2g/l |
| форма | Powder |
| Удельный вес | 2.96 |
| цвет | White |
| Запах | wh. to sl. yel.-wh. powd. or crystals, odorless, tasteless |
| Растворимость в воде | Soluble in water. |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,1706 |
| Пределы воздействия | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 10 mg/m3; TWA 5 mg/m3 |
| Стабильность | Stable. Incompatible with aluminium, strong acids. |
| LogP | -1.031 (est) |
| Справочник по базе данных CAS | 7778-18-9(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | CALCIUM SULFATE |
| FDA UNII | E934B3V59H |
| Система регистрации веществ EPA | Calcium sulfate (7778-18-9) |
| UNSPSC Code | 41123003 |
| NACRES | SB.52 |
| Коды опасности | T,N,Xi | |||||||||
| Заявления о рисках | 49-42/43-51/53-36/37-68-60-50/53 | |||||||||
| Заявления о безопасности | 53-22-36/37-45-60-24/25-36-26-61 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 10 mg/m3 (total) | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | WS6920000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 9 | |||||||||
| кода HS | 28332980 | |||||||||
| Банк данных об опасных веществах | 7778-18-9(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H350:Может вызывать раковые заболевания.
H411:Токсично для водных организмов с долгосрочными последствиями.
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
H360F:Может отрицательно повлиять на способность к деторождению.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Кальция сульфат химические свойства, назначение, производство
Описание
Calcium sulfate and its hydrates are important industrial compounds that have been used throughout history. Calcium sulfate is obtained naturally from mined gypsum rock, but it also exists in mineral form. Gypsum forms in beds as sedimentary rock when calcium sulfate, which is a natural component of seawater, is deposited as shallow marine water bodies evaporate. Gypsum is a transparent, soft, white mineral and is the most common sulfate mineral.Химические свойства
Calcium sulfate forms white to clear crystals. It is commonly encountered in the anhydrous form or as the dihydrate.Физические свойства
Anhydrous calcium sulfate is a crystalline substance; orthorhombic; the color may vary as white, gray, blue or brick-red; occurs as insoluble anhydrite or porous soluble anhydrite; density 2.96 g/cm3; hardness 3.5 Mohs; insoluble anhydrite is practically insoluble in water (0.21% at 20°C); soluble anhydrite readily absorbs moisture and is soluble in water.Hemihydrate is a white fine powder; sparingly soluble in water (3g/L at 25°C); combines with water, setting to a hard mass.
Dihydrate may occur as lumps or powder; density 2.32 g/cm3; partially loses water on heating at 100°C; slightly soluble in water (2.4 g/L at 25°C); KSP =2.4x10-5; almost insoluble in organic solvents.
История
Gypsum’s use dates back to prehistoric times. Archeological evidence shows that it was mined from caves and used to paint ancient gravestones. The earliest evidence of its use as a building material dates from 6000 b.c.e. in the southwest Asian areas of ancient Anatolia and Syria. Egyptians used gypsum and plaster in their buildings and monuments, with both found in the Great Pyramids built around 3700 b.c.e. Calcium sulfate has been used for more than 2,000 years in China to produce tofu. The word gypsum comes from the Greek word for chalk, gypsos. The Greek natural philosopher Theophrastus (371–286 b.c.e.) referred to gypsum in his writings. Gypsum was extensively used in Roman times and throughout the Middle Ages.In the 1700s, Paris was a leading plaster center, with most of its buildings made using plaster. After the fire of London in 1666 destroyed 80% of the city, the king of France ordered all wooden houses in France to be covered with plaster as protection against fire. The hemihydrate form got the name plaster of Paris from the extensive gypsum deposits quarried in the Montmartre district of Paris. In 1765 and 1766, Antoine Lavoisier (1743–1794) presented papers to the French Academy of Science on gypsum that explained the setting of plaster. Lavoisier determined that gypsum is a hydrated salt and that set plaster occurs when the hemihydrate form rehydrates back to gypsum.
Использование
The largest use of calcium sulfate is in the construction industry, which accounts for more than 90% of its production.Gypsum has hundreds of other applications outside the construction industry. It can be used agriculturally for several purposes: to supply calcium and sulfur to soils, to balance pH, and to condition soil. Food grade calcium sulfate is used as a calcium supplement in enriched foods such as flour, cereals, and baked goods. It is used as a gelling and firming agent with canned vegetables. Calcium sulfate is the most common tofu coagulant. The positively charged calcium ion in calcium sulfate attracts the negatively charged groups in protein molecules, causing thermally denatured proteins to coagulate. Anhydrous calcium sulfate is used as a filler to whiten food and consumer products such as frostings, ice creams, paper, paints, and toothpaste. Powdered gypsum can be used as a chalk for marking athletic fields and other large areas.Определение
anhydrite: An important rockforminganhydrous mineral form ofcalcium sulphate, CaSO4. It is chemicallysimilar to gypsum but isharder and heavier and crystallizes inthe rhombic form (gypsum is monoclinic).Under natural conditions anhydriteslowly hydrates to formgypsum. It occurs chiefly in whiteand greyish granular masses and isoften found in the caprock of certainsalt domes. It is used as a raw materialin the chemical industry and inthe manufacture of cement and fertilizers.Методы производства
Anhydrous calcium sulfate occurs naturally as the mineral anhydrite. The naturally occurring rock gypsum may be crushed and ground for use as the dihydrate or calcined at 1508℃ to produce the hemihydrate. A purer variety of calcium sulfate may also be obtained chemically by reacting calcium carbonate with sulfuric acid or by precipitation from calcium chloride and a soluble sulfate.Общее описание
Odorless, white powder or colorless, crystalline solid. Crystals sometimes have a blue, gray or reddish tinge or can be brick red. Density: 2.96 g cm-3.Профиль реактивности
CALCIUM SULFATE is non-combustible. Decomposes to give toxic oxides of sulfur, but only at very high temperature (>1500°C). Generally of low reactivity but may act as an oxidizing agent: incompatible with diazomethane, aluminum, and phosphorus. Certain forms of CALCIUM SULFATE react with water; others do not. INSOLUBLE ANHYDRITE or dead-burned gypsum is made by the dehydration of CALCIUM SULFATE dihydrate (gypsum) at high (> 600°C) temperature. At room temperature, insoluble anhydrite dissolves very slowly to the extent of 0.24 g per 100 g of water and does not absorb moisture from the air. SOLUBLE ANHYDRITE, which is obtained by heating CALCIUM SULFATE dihydrate at a temperature below 300°C, has a high affinity for water and is used as a desiccant. Soluble anhydrite absorbs water to form CALCIUM SULFATE hemihydrate (Plaster of Paris).Угроза здоровью
Calcium sulfate is considered to be a nuisance dust.There have been no reports of adverse effects in humans exposed to calcium sulfate. Excessive concentrations would be expected to cause reduced visibility and skin and upper respiratory tract irritation.1 One report on gypsum miners attributed adverse effects to respirable quartz rather than calcium sulfate.
Фармацевтические приложения
Calcium sulfate dihydrate is used in the formulation of tablets and capsules. In granular form it has good compaction properties and moderate disintegration properties.Calcium sulfate hemihydrate, is used in the preparation of plaster of Paris bandage, which is used for the immobilization of limbs and fractures; it should not be used in the formulation of tablets or capsules.
Anhydrous calcium sulfate is hygroscopic and is used as a desiccant. The uptake of water can cause the tablets to become very hard and to fail to disintegrate on storage. Therefore, anhydrous calcium sulfate is not recommended for the formulation of tablets, capsules, or powders for oral administration.
Therapeutically, calcium sulfate is used in dental and craniofacial surgical procedures.
Сельскохозяйственное использование
Anhydrite is a naturally occurring, solid, white mineral called anhydrous calcium sulphate (CaSO4). It differs from gypsum in hardness and in hydration. It is used as a raw material in the chemical industry and in the manufacture of fertilizers and cement.Профиль безопасности
A nuisance dust. Reacts violently with aluminum when heated. Mixtures with diazomethane react exothermically and eventually explode. Mixtures with phosphorus ignite at high temperatures. When heated to decomposition it emits toxic fumes of SO,, See also CALCIUM COMPOUNDS and SULFATES.Безопасность
Calcium sulfate dihydrate is used as an excipient in oral capsule and tablet formulations. At the levels at which it is used as an excipient, it is generally regarded as nontoxic. However, ingestion of a sufficiently large quantity can result in obstruction of the upper intestinal tract after absorption of moisture.Owing to the limited intestinal absorption of calcium from its salts, hypercalcemia cannot be induced even after the ingestion of massive oral doses.
Calcium salts are soluble in bronchial fluid. Pure salts do not induce pneumoconiosis.
Возможный контакт
Calcium sulfate is used as a pigment; in Portland cement, in tiles and plaster; in polishing powders, a filler in paints and paper coatings; in the drying of gases and liquids; a soil conditioner; in molds and surgical casts; in wallboard, and many others.хранилище
Calcium sulfate is chemically stable. Anhydrous calcium sulfate is hygroscopic and may cake on storage. Store in a well-closed container in a dry place, avoiding heat.Перевозки
Calcium sulfate is a “NONREGULATED MATERIAL.”Несовместимости
In the presence of moisture, calcium salts may be incompatible with amines, amino acids, peptides, and proteins, which may form complexes. Calcium salts will interfere with the bioavailability of tetracycline antibiotics. It is also anticipated that calcium sulfate would be incompatible with indomethacin, aspirin, aspartame, ampicillin, cephalexin, and erythromycin since these materials are incompatible with other calcium salts.Calcium sulfate may react violently, at high temperatures, with phosphorus and aluminum powder; it can react violently with diazomethane.
Утилизация отходов
LandfillingРегуляторный статус
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules, sustained release, tablets). Included in nonparenteral medicines licensed in the UK and Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Кальция сульфат запасные части и сырье
Кальция сульфат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| 18017610038 | CHINA | 3619 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |
Кальция сульфат Обзор)
1of4