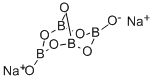
Натрий тетраборат
- английское имяSodium tetraborate
- CAS №1330-43-4
- CBNumberCB6233954
- ФормулаB4Na2O7
- мольный вес201.22
- EINECS215-540-4
- номер MDLMFCD00081185
- файл Mol1330-43-4.mol
| Температура плавления | 741 °C (lit.) |
| Температура кипения | 1575°C |
| Плотность накопления | 700kg/m3 |
| плотность | 2.367 g/mL at 25 °C (lit.) |
| давление пара | 7.3 hPa (1200 °C) |
| показатель преломления | 1.501 |
| Fp | 1575°C |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | H2O: 0.1 M at 20 °C, clear, colorless |
| форма | Solid |
| Удельный вес | 2.367 |
| цвет | White |
| РН | 9.0-10.5 (25℃, 0.1M in H2O) |
| Растворимость в воде | 26 g/L (20 ºC) |
| Чувствительный | Hygroscopic |
| λмакс | λ: 260 nm Amax: ≤0.020 λ: 280 nm Amax: ≤0.015 |
| Мерк | 14,8590 |
| Пределы воздействия | ACGIH: TWA 2 mg/m3; STEL 6 mg/m3 NIOSH: TWA 1 mg/m3 |
| Стабильность | Stable. Incompatible with powdered metals. |
| ИнЧИКей | UQGFMSUEHSUPRD-UHFFFAOYSA-N |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | SODIUM BORATE |
| FDA UNII | 8191EN8ZMD |
| Справочник по химии NIST | Sodium borate(1330-43-4) |
| Система регистрации веществ EPA | Sodium tetraborate (1330-43-4) |
| UNSPSC Code | 12352302 |
| NACRES | NB.21 |
| Коды опасности | Xn,Xi,T | |||||||||
| Заявления о рисках | 62-63-36/38-36/37/38-61-60 | |||||||||
| Заявления о безопасности | 36/37-24/25-26-36-23-45-53 | |||||||||
| РИДАДР | UN 1760 8/PG 2 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 1 mg/m3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | ED4588000 | |||||||||
| F | 34 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28401100 | |||||||||
| Банк данных об опасных веществах | 1330-43-4(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
Натрий тетраборат химические свойства, назначение, производство
Химические свойства
Sodium borate, Na2B407.10H20, also known as sodium tetraborate and sodium pyrobomte, is a white crystalline powder that melts at 120°C (248 °F). Sodium borate in its natural impure form is also known as borax, Borax is a noncombustible (an inherent fire retardant), bluish-gray or green, odorless crystalline powder or granules. Sodium borate is used in glass and ceramic enamel mixes,detergents, fertilizers, pharmaceuticals, and photograph.Использование
Sodium Tetraborate, is an important boron compound, which has a wide variety of applications. It is a component of many detergents, cosmetics, and enamel glazes. It is also used to make buffer solutions in biochemistry, as a fire retardant, as an anti-fungal compound for fiberglass, as a flux in metallurgy, neutron-capture shields for radioactive sources, a texturing agent in cooking, and as a precursor for other boron compounds.Методы производства
Anhydrous borax is produced from borax through high temperature fusion. On cooling, the clear, glass-like material is ground into fine white granules. Because of its higher bulk density, it is preferred where storage space is limited. It is used principally in the manufacture of glass, ceramics, and enamel.Общее описание
Pale yellow solid or thick liquid with a faint odor of detergent. Mixes with water. Soap bubbles may be produced.Реакции воздуха и воды
Water soluble.Профиль реактивности
SODIUM PEROXOBORATE is incompatible with the following: Zirconium, strong acids, metallic salts . The true peroxoborate has been reported to detonate on light friction. The common "tetrahydrate" is not a peroxoborate, Sodium tetraborate is relatively stable under mild grinding with other substances.Угроза здоровью
No adverse effects from inhaling borax have been reported. Ingestion may cause acute or chronic effects; initial symptoms are nausea, vomiting, and diarrhea; these may be followed by weakness, depression, headaches, skin rashes, drying skin, cracked lips, and loss of hair; shock may follow ingestion of large doses and may interfere with breathing. Eye contact with powder or solutions may cause irritation; no chronic effects have been recognized, but continued contact should be avoided. Local skin irritation may result from contact with powder or strong solutions; the latter may cause chronic dermatitis on prolonged contact, and if skin is broken, enough boron may be absorbed to cause boron poisoning (symptoms are similar to those for ingestion).Сельскохозяйственное использование
Sodium tetraborate, also called borax, sodium borate, sodium pyroborate and sodium tetraborate decahydrate (Na2B4O7.10H2O) is a type of borate, and is used as a fertilizer to reduce boron deficiency. It is a white salt, finely ground for fertilizer application.Sodium tetraborate(Na2B4O7) and sodium metaborate (Na2B2O4) are non-selective, taken up by roots, and have an unknown mechanism of action. Boron accumulates in reproductive structures after translocation from roots. Boron compounds are used for long-term, nonselective weed control in industrial and power line areas in combination with triazine and urea herbicides.
Профиль безопасности
A nuisance dust. Experimental reproductive effects. When heated to decomposition it emits toxic vapors of B.Возможный контакт
Borax is used as a soldering flux, preservative against wood fungus; and as an antiseptic. Used in ant poisons, for fly control around refuse and manure piles, as a larvicide. It is used in the manufacture of enamels and glazes, fiberglass insulation; sodium perborate bleach; in tanning, cleaning compounds; for fireproofing fabrics and wood; and in artificial aging of wood.Методы очистки
Most of the water of hydration is removed from the decahydrate (see below) by evacuation at 25o for three days, followed by heating to 100o and evacuation with a high-speed diffusion pump. The dried sample is then heated gradually to fusion (above 966o), allowed to cool gradually to 200o, then tranferred to a desiccator containing P2O5 [Grenier & Westrum J Am Chem Soc 78 6226 1956]. [Becher in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 794-795 1963.]Несовместимости
Dissolves in water forming a basic solution. Boron dust may form explosive mixture with air. Contact with strong oxidizers may be violent. Boron is incompatible with ammonia, bromine tetrafluoride, cesium carbide, chlorine, fluorine, interhalogens, iodic acid, lead dioxide, nitric acid, nitric oxide, nitrosyl fluoride, nitrous oxide, potassium nitrite, rubidium carbide, silver fluoride.Утилизация отходов
Borax, dehydrated: The material is diluted to the recommended provisional limit (0.10 mg/L) in water. The pH is adjusted to between 6.5 and 9.1 and then the material can be discharged into sewers or natural streams.Натрий тетраборат запасные части и сырье
сырьё
запасной предмет
Натрий тетраборат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-021-62885108 +8613917661608 |
China | 2068 | 57 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 |