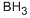
Бор
- английское имяBoron
- CAS №7440-42-8
- CBNumberCB0299730
- ФормулаB
- мольный вес10.81
- EINECS231-151-2
- номер MDLMFCD00134034
- файл Mol7440-42-8.mol
| Температура плавления | 2300°C |
| Температура кипения | 2550°C |
| плотность | 2.34 g/mL at 25 °C (lit.) |
| температура хранения | no restrictions. |
| растворимость | H2O: soluble |
| форма | Powder |
| цвет | Brown or dark |
| Удельный вес | 2.34~2.37 |
| удельное сопротивление | 1.5E12 μΩ-cm, 20 °C |
| Растворимость в воде | insoluble H2O [MER06] |
| Кристальная структура | Trigonal (rhombohedral) a = 1017 pm α = 65°12' hR105, R3m, β-B type |
| Мерк | 13,1333 |
| Пределы воздействия | ACGIH: TWA 2 mg/m3; STEL 6 mg/m3 |
| Стабильность | Stable. Substances to be avoided include strong oxidizing agents and strong acids. May decompose on exposure to air - store under nitrogen. Highly flammable. |
| ИнЧИКей | UORVGPXVDQYIDP-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 7440-42-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | N9E3X5056Q |
| Справочник по химии NIST | Boron(7440-42-8) |
| Система регистрации веществ EPA | Boron (7440-42-8) |
| Коды опасности | Xn,F | |||||||||
| Заявления о рисках | 22-11-63-62 | |||||||||
| Заявления о безопасности | 16-24/25-45-36/37/39-27-26 | |||||||||
| РИДАДР | UN 3178 4.1/PG 2 | |||||||||
| WGK Германия | - | |||||||||
| RTECS | ED7350000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28045000 | |||||||||
| Банк данных об опасных веществах | 7440-42-8(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Бор химические свойства, назначение, производство
Описание
Boron was discovered by Sir Humphry Davy and J.L. Gay-Lussac in 1808. It is a trivalent non-metallic element that occurs abundantly in the evaporite ores borax and ulexite. Boron is never found as a free element on Earth. Boron appears as charcoal-grey pieces or black powder or as crystalline; is a very hard, black material with a high melting point; and exists in many polymorphs.
Boron has several forms, and the most common one is amorphous boron, a dark powder, non-reactive to oxygen, water, acids, and alkalis. It reacts with metals to form borides. Boron is an essential plant micronutrient. Sodium borate is used in biochemical and chemical laboratories to make buffers. Boric acid is produced mainly from borate minerals by the reaction with sulphuric acid. Boric acid is an important compound used in textile products. The most economically important compound of boron is sodium tetraborate decahydrate or borax, used for insulating fibreglass and sodium perborate bleach. Compounds of boron are used in organic synthesis, in the manufacture of a particular type of glasses, and as wood preservatives. Boron filaments are used for advanced aerospace structures, due to their high strength and light weight.
Химические свойства
Boron is a yellow or brownish-black powder and may be either crystalline or amorphous. It does not occur free in nature and is found in the minerals borax, colemanite, boronatrocalcite, and boracite. It is insoluble in water but soluble in nitric and sulfuric acids. It is insoluble in cold water, hot water, diethyl ether, and alcohol. If finely divided, it is soluble in most molten metals such as copper, iron, magnesium, aluminum, and calcium. Borates are relatively soluble in water.Физические свойства
Boron has only three electrons in its outer shell, which makes it more metal than nonmetal.Nonmetals have four or more electrons in their valence shell. Even so, boron is somewhatrelated to metalloids and also to nonmetals in period 2.It is never found in its free, pure form in nature. Although less reactive than the metalswith fewer electrons in their outer orbits, boron is usually compounded with oxygen andsodium, along with water, and in this compound, it is referred to as borax. It is also found asa hard, brittle, dark-brown substance with a metallic luster, as an amorphous powder, or asshiny-black crystals.
Its melting point is 2,079°C, its boiling point is 2,550°C, and its density is 2.37 g/cm3.
Изотопы
There are a total of 13 isotopes of boron, two of which are stable. The stableisotope B-10 provides 19.85% of the element’s abundance as found in the Earth’s crust,and the isotope B-11 provides 80.2% of boron’s abundance on Earth.Происхождение имени
It is named after the Arabic word bawraq, which means “white borax.”Вхождение
Boron is the 38th most abundant element on Earth. It makes up about 0.001% of theEarth’s crust, or 10 parts per million, which is about the same abundance as lead. It is notfound as a free element in nature but rather in the mineral borax, which is a compound ofhydrated sodium, hydrogen, and water. Borax is found in salty lakes, dry lake-beds, or alkalisoils. Other naturally occurring compounds are either red crystalline or less dense, dark-brownor black powder.Boron is also found in kernite, colemanite, and ulexite ores, and is mined in many countries,including the western United States.
Характеристики
Boron is a semimetal, sometimes classed as a metallic or metalloid or even as a nonmetal.It resembles carbon more closely than aluminum. Although it is extremely hard in its purified form—almost as hard asdiamonds—it is more brittle than diamonds, thus limiting its usefulness. It is an excellentconductor of electricity at high temperatures, but acts as an insulator at lower temperatures.Использование
Boron has found many uses and has become an important industrial chemical. Boron is used as an alloy metal, and when combined with other metals, it imparts exceptional strength to those metals at high temperatures. It is an excellent neutron absorber used to capture neutrons in nuclear reactors to prevent a runaway fission reaction. As the boron rods are lowered into the reactor, they control the rate of fission by absorbing excess neutrons. Boron is also used as an oxygen absorber in the production of copper and other metals, Boron finds uses in the cosmetics industry (talc powder), in soaps and adhesives, and as an environmentally safe insecticide. A small amount of boron is added as a dope to silicon transistor chips to facilitate or impede the flow of current over the chip. Boron has just three valence electrons; silicon atoms have four. This dearth of one electron in boron s outer shell allows it to act as a positive hole in the silicon chip that can be filled or left vacant, thus acting as a type of switch in transistors. Many of today s electronic devices depend on these types of doped-silicon semiconductors and transistors. Boron is also used to manufacture borosilicate glass and to form enamels that provide a protective coating for steel. It is also used as medication for relief of the symptoms of arthritis. Due to boron s unique structure and chemical properties, there are still more unusual compounds to be explored.Подготовка
Boron may be prepared by several methods, such as chemical reduction of boron compounds, electrolytic reduction in nonaqueous phase, or by thermal decomposition. Many boron compounds including boron oxides, borates, boron halides, borohydrides, and fluoroborates can be reduced to boron by a reactive metal or hydrogen at high temperatures:B2O3 + 3Ca → 2B + 3CaO
The metal is obtained as a black amorphous product.
2BCl3 + 3H2 → 2B + 6HCl
High purity grade boron may be prepared by such hydrogen reduction at high temperatures using a hot filament.
Electrolytic reduction and thermal decomposition have not yet been applied in large scale commercial methods. Electrolysis of alkali or alkaline earth borates produces boron in low purity. Electrolytic reduction of fused melts of boron trioxide or potassium tetrafluroborate in potassium chloride yield boron in high purity. Also, boron tribromide or boron hydrides may be thermally dissociated by heating at elevated temperatures.
Impurities from boron may be removed by successive recrystallization or volatilization at high temperatures. Removal of certain impurities such as oxygen, nitrogen, hydrogen or carbon from boron are more difficult and involve more complex steps.
Методы производства
Until the late 1990s elemental boron had not found widespread use in industry, where cost of production was a major obstacle. Now, there is increasing use as new applications for the element are developed in material composites and use in nanotechnology.Определение
boron: Symbol B. An element ofgroup 13 (formerly IIIB) of the periodictable; a.n. 5; r.a.m. 10.81; r.d.2.34–2.37 (amorphous); m.p. 2300°C;b.p. 2550°C. It forms two allotropes;amorphous boron is a brown powderbut metallic boron is black. Themetallic form is very hard (9.3 onMohs’ scale) and is a poor electrical conductor at room temperature. Atleast three crystalline forms are possible;two are rhombohedral and theother tetragonal. The element isnever found free in nature. It occursas orthoboric acid in volcanic springsin Tuscany, as borates in kernite(Na2B4O7.4H2O), and as colemanite(Ca2B6O11.5H2O) in California. Samplesusually contain isotopes in theratio of 19.78% boron–10 to 80.22%boron–11. Extraction is achieved byvapour-phase reduction of borontrichloride with hydrogen on electricallyheated filaments. Amorphousboron can be obtained by reducingthe trioxide with magnesium powder.Boron when heated reacts withoxygen, halogens, oxidizing acids,and hot alkalis. It is used in semiconductorsand in filaments for specializedaerospace applications.Amorphous boron is used in flares,giving a green coloration. The isotopeboron–10 is used in nuclear reactorcontrol rods and shields. Theelement was discovered in 1808 bySir Humphry Davy and by J. L. Gay-Lussac and L. J. Thenard.Опасность
Powdered or fine dust of elemental boron is explosive in air and toxic if inhaled. Several ofthe compounds of boron are very toxic if ingested or if they come in contact with the skin. Thisis particularly true of the boron compounds used for strong insecticides and herbicides.Угроза здоровью
Boron has been studied extensively for its nutritional importance in animals and humans. There is a growing body of evidence that boron may be an essential element in animals and humans. Many nutritionists believe that people would benefi t from more boron and many popular multivitamins, such as centrum, in the diet. The adverse health effects of boron on humans is limited. However, ingestion/inhalation causes irritation to the mucous membrane and boron poisoning. Short-term exposures to boron in work areas are known to cause irritation of the eye, the upper respiratory tract, and the naso-pharynx, but the irritation disappears with the stoppage of further exposure. Ingestion of large amounts of boron (about 30 g of boric acid)over short periods of time is known to affect the stomach, intestines, liver, kidney, and brain and can eventually lead to death in exposed people.Промышленное использование
Boron (symbol B) is a metallic element closelyresembling silicon. Boron has a specific gravityof 2.31, a melting point of about 2200°C, anda Knoop hardness of 2700 to 3200, equal to aMohs hardness of about 9.3. At 600°C, boronignites and burns with a brilliant green flame.Minute quantities of boron are used in steelsfor case hardening by the nitriding process toform a boron nitride, and in other steels toincrease hardenability, or depth of hardness. Inthese boron steels, as little as 0.003% is beneficial,forming an iron boride, but with largeramounts the steel becomes brittle and susceptibleto hot-short unless it contains titanium orsome other element to stabilize the carbon . Incast iron, boron inhibits graphitization and alsoserves as a deoxidizer. It is added to iron andsteel in the form of ferroboron.Boron compounds are employed for fluxesand deoxidizing agents in melting metals, andfor making special glasses. Boron, like siliconand carbon, has an immense capacity for formingcompounds, although it has a differentvalence. The boron atom appears to have a lenticularshape, and two boron atoms can make astrong electromagnetic bond, with the boronacting like carbon but with a double ring.
Возможный контакт
Boron is used in metallurgy as a degasifying agent and is alloyed with aluminum, iron, and steel to increase hardness. It is also a neutron absorber in nuclear reactors. Boron is frequently encountered in a variety of chemical formulations including boric acid, various borate salts, borax, and boron soil supplements.Перевозки
Boron powder or dust: UN3178 Flammable solid, inorganic, Hazard Class: 4.1; Labels: 4.1—Flammable solid.Структура и конформация
The space lattice of Boron belongs to the tetragonal system with lattice constants a=0.873 nm, c=1.013 nm (c=0.503 nm is also reported). The rhombohedron system is also formed. The rhombohedron is stable near the melting point.Energy gap: Eg=1.0–1.5 eV
Activation energy : 1.39±0.05 eV
Electron mobility: μe=0.9 cm2 /V s (300 K, 1.8×1016 cm-3 )
Несовместимости
Boron dust may form explosive mixture in air. Contact with strong oxidizers may cause explosions. Violent reaction (possible explosion) with concentrated nitric acid, hydrogen iodide; silver fluoride. Boron is incompatible with ammonia, bromine tetrafluoride, cesium carbide, chlorine, fluorine, interhalogens, iodic acid, lead dioxide, nitric acid, nitrosyl fluoride, nitrous oxide, potassium nitrite, rubidium carbide. Reacts exothermically with metals at high temperature above 900° C.Утилизация отходов
Dispose of contents and container to an approved waste disposal plant. All federal, state, and local environmental regulations must be observed.Меры предосторожности
Elemental boron is non-toxic and common boron compounds, such as borates and boric acid, have low toxicity (approximately similar to table salt with the lethal dose being 2–3 g/kg) and do not require special precautions while handling. Some of the more exotic boron hydrogen compounds, however, are toxic as well as highly flammable and do require special care when handlingБор запасные части и сырье
Бор поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-85511178 +86-85511178 |
China | 35451 | 58 | ||
| +undefined-86-1523780-4566 +undefined15237804566 |
China | 511 | 58 | ||
| +86-024-23769576 +86-15040101888 |
China | 255 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 2989 | 58 | ||
| +8613288715578 | China | 549 | 58 | ||
| +86-0531-8875-2665 +8613153039501 |
China | 531 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21670 | 55 | ||
| 008657128800458; +8615858145714 |
China | 9337 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29897 | 58 | ||
| 18871490254 | CHINA | 28180 | 58 |