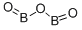
Бора оксид
- английское имяBoron oxide
- CAS №1303-86-2
- CBNumberCB1264526
- ФормулаB2O3
- мольный вес69.62
- EINECS215-125-8
- номер MDLMFCD00011315
- файл Mol1303-86-2.mol
| Температура плавления | 450 °C(lit.) | ||||||||||||||
| Температура кипения | 1860 °C | ||||||||||||||
| плотность | 2.46 g/mL at 25 °C(lit.) | ||||||||||||||
| Плотность накопления | 900-1100kg/m3 | ||||||||||||||
| плотность пара | >1 (vs air) | ||||||||||||||
| давление пара | 1Pa | ||||||||||||||
| Fp | 1860°C | ||||||||||||||
| температура хранения | Inert atmosphere,Room Temperature | ||||||||||||||
| растворимость | 36g/l | ||||||||||||||
| форма | pellets | ||||||||||||||
| Удельный вес | 2.46 +/- 0.01 | ||||||||||||||
| цвет | White | ||||||||||||||
| РН | 4 (10g/l, H2O, 25℃) | ||||||||||||||
| Запах | Odorless | ||||||||||||||
| Биологические источники | human blood | ||||||||||||||
| Растворимость в воде | 36 g/L (25 ºC) | ||||||||||||||
| Чувствительный | Hygroscopic | ||||||||||||||
| Мерк | 14,1337 | ||||||||||||||
| crystal system | Three sides | ||||||||||||||
| Space group | P31 | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Пределы воздействия | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3 NIOSH: IDLH 2000 mg/m3; TWA 10 mg/m3 |
||||||||||||||
| Стабильность | Stable. Moisture sensitive. Incompatible with water. | ||||||||||||||
| ИнЧИКей | JKWMSGQKBLHBQQ-UHFFFAOYSA-N | ||||||||||||||
| Справочник по базе данных CAS | 1303-86-2(CAS DataBase Reference) | ||||||||||||||
| Рейтинг продуктов питания EWG | 1 | ||||||||||||||
| FDA UNII | 483W67CPF4 | ||||||||||||||
| Справочник по химии NIST | Diboron trioxide(1303-86-2) | ||||||||||||||
| Система регистрации веществ EPA | Boric oxide (1303-86-2) | ||||||||||||||
| UNSPSC Code | 41116105 | ||||||||||||||
| NACRES | NB.21 |
| Коды опасности | Xi,T | |||||||||
| Заявления о рисках | 36/37/38-61-60 | |||||||||
| Заявления о безопасности | 26-37/39-45-53 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 10 mg/m3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | ED7900000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28100010 | |||||||||
| Банк данных об опасных веществах | 1303-86-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 3150 mg/kg | |||||||||
| ИДЛА | 2,000 mg/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H360D:Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Бора оксид химические свойства, назначение, производство
Химические свойства
Boron oxide is a noncombustible, colorless, semitransparent lumps or hard, white, odorless crystals, with slightly bitter taste.
Физические свойства
Colorless glassy solid or vitreous crystal; hexagonal crystal system; slightly bitter taste; hygroscopic; density 2.55 g/cm3; melts at 450°C; vaporizes at 1,500°C; slightly soluble in cold water (3.3%), soluble in alcohol and boiling water (20%).Использование
Boron oxide was used as the intermediate glass layer at a bonding temperature of 450°C. In preparation of fluxes; component of enamels and glass; catalyst in organic reaction.In metallurgy; in analysis of silicates to determine SiO2 and alkalies; in blowpipe analysis.
Подготовка
Boric oxide is produced by treating borax with sulfuric acid in a fusion furnace. At temperatures above 750°C, the molten boric acid layer separates out from sodium sulfate. It then is decanted, cooled, and obtained in 96-97% purity. Boric acid above 99% purity may be obtained by fusing granular material.Boric oxide may be prepared by heating boric acid.
2B(OH)3 → B2O3 + 3H2O
Методы производства
Boric oxide is produced by thermal fusion of boric acid, forming a clear transparent glass-like solid that is subsequently ground into white vitreous granules. It is used principally in the manufacture of glass and vitreous products.Общее описание
Colorless, semi-transparent glassy lumps or hard white odorless crystals. Mp 450°C; bp: 1860°C. Density: 2.46 g cm-3. Moderately soluble in water. Used as an insecticide; as the starting material for the synthesis of other boron compounds; as a fluxing agent in enamels and glasses; and in mixture with 2-6% boron nitride, as a bonding agent in the hot isostatic pressing of boron nitride ceramics.Профиль реактивности
Boron oxide is non-combustible. Of generally low chemical reactivity. Reacts exothermically but slowly with water to form boric acid, a weak acid. Reacts exothermically with strong bases. May react with strong reducing agents such as metal hydrides, metal alkyls to generate flammable or explosive gases. May react violently on contact with bromine pentafluoride. Corrosive to metals in the presence of air.Опасность
Eye and upper respiratory tract irritant.Угроза здоровью
Boron oxide is an eye and respiratory irritant. In 113 workers exposed to boron oxide and boric acid dusts, there were statistically significant increases in symptoms of eye irrita- tion; dryness of the mouth, nose, and throat; sore throat; and productive cough compared with controls. The mean exposure level was 4.1mg/m3 , with a range of 1.2–8.5mg/m3 . Exposures may occasionally have exceeded 10 mg/m3 . Because of mixed exposures, the study does not indicate whether boron oxide or boric acid dust is more important in causing symp- toms, nor does it indicate the minimum duration of exposure necessary to produce symptoms.Excessive absorption of boron oxide may lead to cardiovascular collapse, alterations in temperature regulation, and coma.
Возможный контакт
Boron oxide is used in glass manufacture and the production of other boron compounds. It is used in fluxes, enamels, drying agents, and as a catalyst.Перевозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required.Несовместимости
Incompatible with bromine pentafluoride, calcium oxide. Reacts slowly with water, forming boric acid. Reacts exothermically with alkaline material and strong bases. May react with strong reducing agents such as metal hydrides, metal alkyls to generate flammable or explosive gases. May react violently on contact with bromine pentafluoride. Corrosive to metals in the presence of air.Бора оксид запасные части и сырье
Бора оксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-025-86655873 +8613962173137 |
China | 199 | 55 | |
| +8617732866630 | China | 8773 | 58 | |
| +86-29-81148696 +86-17392712697 |
China | 3889 | 58 | |
| +8613288715578 | China | 12554 | 58 | |
| +86-17331933971 | China | 2472 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-0531-8875-2665 +8613153039501 |
China | 661 | 58 | |
| +86-15350851019; +8615350851019 |
China | 1000 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21622 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2988 | 55 |