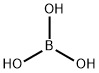
Борная кислота
- английское имяOrthoboric acid
- CAS №10043-35-3
- CBNumberCB6128144
- ФормулаBH3O3
- мольный вес61.83
- EINECS233-139-2
- номер MDLMFCD00236358
- файл Mol10043-35-3.mol
| Температура плавления | 160 °C (dec.) (lit.) |
| Температура кипения | 219-220 °C (9.7513 mmHg) |
| плотность | 1.440 g/cm3 |
| давление пара | 2.6 mm Hg ( 20 °C) |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | H2O: soluble |
| форма | working solution |
| пка | 8.91±0.43(Predicted) |
| Удельный вес | 1.435 |
| цвет | ≤10(APHA) |
| Запах | Odorless |
| Водородный показатель | 3.8 - 4.8 |
| РН | 3.6-4.4 (25℃, saturated solution in H2O) |
| Растворимость в воде | 49.5 g/L (20 ºC) |
| Чувствительный | Hygroscopic |
| λмакс | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.05 |
| Мерк | 14,1336 |
| БРН | 1697939 |
| Пределы воздействия | ACGIH: TWA 2 mg/m3; STEL 6 mg/m3 |
| ИнЧИКей | KGBXLFKZBHKPEV-UHFFFAOYSA-N |
| LogP | -1.09 at 22℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | BORIC ACID |
| Справочник по базе данных CAS | 10043-35-3(CAS DataBase Reference) |
| FDA UNII | R57ZHV85D4 |
| Справочник по химии NIST | B(OH)3(10043-35-3) |
| Система регистрации веществ EPA | Orthoboric acid (10043-35-3) |
| Коды опасности | Xi,T,Xn | |||||||||
| Заявления о рисках | 36/37/38-60-63-62-61 | |||||||||
| Заявления о безопасности | 26-36-53-45-37/39-36/37/39-22-24/25-23 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | ED4550000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28100090 | |||||||||
| Банк данных об опасных веществах | 10043-35-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 5.14 g/kg (Smyth). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Борная кислота химические свойства, назначение, производство
Химические свойства
White powder or granules and odorless. It is incompatible with potassium, acetic anhydride, alkalis, carbonates, and hydroxides. Boric acid has uses in the production of textile fiberglass, flat panel displays, and eye drops. Boric acid is recognized for its application as a pH buffer and as a moderate antiseptic agent and emulsifier.Физические свойства
Colorless, transparent triclinic crystal or white granule or powder; density 1.435 g/cm3; melts at 171°C under normal heating; however, slow heating causes loss of water; sparingly soluble in cold water (4.7% at 20°C); pH of 0.1M solution 5.1; readily dissolves in hot water (19.1% at 80°C and 27.5% at 100°C); also soluble in lower alcohols and moderately soluble in pyridine.Использование
For weatherproofing wood and fireproofing fabrics; as a preservative; manufacture of cements, crockery, porcelain, enamels, glass, borates, leather, carpets, hats, soaps, artificial gems; in nickeling baths; cosmetics; printing and dyeing, painting; photography; for impregnating wicks; electric condensers; hardening steel. Also used as insecticide for cockroaches and black carpet beetles.Подготовка
Boric acid is produced from borax, colemanite, or other inorganic borates by reaction with sulfuric acid or hydrochloric acid, and cooling the solution to proper temperature:Na2B4O7 ? 10Η2Ο + H2SO4 → 4H3BO3 + Na2SO4 + 5H2O
It also may be prepared by extraction of weak borax brine with a kerosene solution of an aromatic diol, such as 2-ethyl-1,3-hexanediol or 3-chloro- 2-hydroxy-5-(1,1,3,3-tetramethylbutyl)benzyl alcohol. The diol-borate chelate formed separates into a kerosene phase. Treatment with sulfuric acid yields boric acid which partitions into aqueous phase and is purified by recrystallization.
Методы производства
Boric acid occurs naturally as the mineral sassolite. However, the majority of boric acid is produced by reacting inorganic borates with sulfuric acid in an aqueous medium. Sodium borate and partially refined calcium borate (colemanite) are the principal raw materials. When boric acid is made from colemanite, the fineground ore is vigorously stirred with mother liquor and sulfuric acid at about 908℃. The by-product calcium sulfate is removed by filtration, and the boric acid is crystallized by cooling the filtrate.Всемирная организация здравоохранения(ВОЗ)
Boric acid and some borates were formerly extensively used as disinfectants and antiinflammatory agents. By the late 1960s an association between the death of many infants and application of high concentrations of boric acid contained in topical preparations used in the treatment of napkin rash had been established. This led to the restriction of the use of boric acid in pharmaceutical preparations by many regulatory authorities. In some countries it is now permitted only as an ingredient in ophthalmological preparations.Общее описание
Boric acid is a weak monobasic acid, it accepts OH- ions, hence is a Lewis acid. In boric acid, B is sp2 hybridized, forming a planar triangle structure. The principal oxide of boron, B2O3, is obtained as a vitreous solid by dehydration of boric acid at red heat.Опасность
Toxic via ingestion. Use only weak solu- tions. Irritant to skin in dry form.Фармацевтические приложения
Boric acid is used as an antimicrobial preservative in eye drops, cosmetic products, ointments, and topical creams. It is also used as an antimicrobial preservative in foods.Boric acid and borate have good buffering capacity and are used to control pH; they have been used for this purpose in external preparations such as eye drops.
Boric acid has also been used therapeutically in the form of suppositories to treat yeast infections. In dilute concentrations it is used as a mild antiseptic, with weak bacteriostatic and fungistatic properties, although it has generally been superseded by more effective and less toxic disinfectants.
Возможный контакт
Boric acid is a fireproofing agent for wood; a preservative, and an antiseptic. It is used in the manufacture of glass, pottery, enamels, glazes, cosmetics, cements, porcelain, borates, leather, carpets, hats, soaps; artificial gems; in tanning leather; printing, dyeing, painting, and photography.хранилище
Boric acid is hygroscopic and should therefore be stored in an airtight, sealed container. The container must be labeled ‘Not for Internal Use’.Перевозки
UN 3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required.Методы очистки
Crystallise the acid three times from H2O (3mL/g) between 100o and 0o, after filtering through sintered glass.Dry it to constant weight over metaboric acid in a desiccator. It is steam volatile. After two recrystallisations of ACS grade. it had Ag at 0.2 ppm. Its solubility (%) in H2O is 2.66 at 0o, 4.0 at 12o and 24 at 80o. At 100o it loses H2O to form metaboric acid (HBO2). When it is heated to redness or slowly to 200o, or over P2O5 in vacuo, it dehydrates to boric anhydride (B2O3) [1303-82-6] to give a white hard glass or crystals with m ~294o.The glass softens on heating and liquefies at red heat. It is an astringent, a fungicide and an antibacterial. [McCulloch J Am Chem Soc 59 2650 1937, Kelly J Am Chem Soc 63 1137 1941, Taylor & Cole J Chem Soc 70 1926, Conti J Soc Chem Ind 44 343T 1925.]Несовместимости
Boric acid decomposes in heat above 100 C, forming boric anhydride and water. Boric acid is hygroscopic; it will absorb moisture from the air. Boric acid aqueous solution is a weak acid; incompatible with strong reducing agents including alkali metals and metal hydrides (may generate explosive hydrogen gas); acetic anhydride, alkali carbonates, and hydroxides. Violent reaction with powdered potassium metal, especially if impacted. Attacks iron in the presence of moisture.Утилизация отходов
Boric acids may be recovered from organic process wastes as an alternative to disposal.Регуляторный статус
Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IV injections; ophthalmic preparations; (auricular) otic solutions; topical preparations). Reported in the EPA TSCA Inventory. In the UK, the use of boric acid in cosmetics and toiletries is restricted. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Борная кислота запасные части и сырье
сырьё
запасной предмет
- Copper(II) borofluoride
- Zinc tetrafluoroborate
- Натрий гидрид
- Бора оксид
- Триизопропаноламин циклич. борат
- Foliar-fertilizer
- Цинка борат
- Трибутилборат
- Ацетонитрильный комплекс трифторида бора
- Натрий тетрафторборат
1of6
Борная кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13734021967 +8613734021967 |
China | 1011 | 58 | ||
| +86-13545906766; +8613545906766 |
China | 265 | 58 | ||
| +8615389281203 | China | 980 | 58 | ||
| 15369953316 +8615369953316 |
China | 2136 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12459 | 58 | ||
| +86-18033737140 +86-17354101231 |
China | 253 | 58 | ||
| +undefined18602966907 | China | 1000 | 58 | ||
| 571-85586718 +8613336195806 |
China | 29798 | 60 | ||
| +86-518-85110578 +8618805133257 |
China | 132 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21670 | 55 |