Висмута оксид
- английское имяBismuth(III) Oxide
- CAS №1304-76-3
- CBNumberCB5716407
- ФормулаBiO3-
- мольный вес256.98
- EINECS215-134-7
- номер MDLMFCD00003462
- файл Mol1304-76-3.mol
химическое свойство
| Температура плавления | 825 °C | ||||||||||||||
| Температура кипения | 1890°C | ||||||||||||||
| Плотность накопления | 1000kg/m3 | ||||||||||||||
| плотность | 8.9 | ||||||||||||||
| Fp | 1890°C | ||||||||||||||
| температура хранения | Store at +5°C to +30°C. | ||||||||||||||
| растворимость | 0.006g/l practically insoluble | ||||||||||||||
| форма | powder | ||||||||||||||
| цвет | Yellow | ||||||||||||||
| Удельный вес | 8.9 | ||||||||||||||
| Запах | Odorless | ||||||||||||||
| Растворимость в воде | INSOLUBLE | ||||||||||||||
| Мерк | 14,1273 | ||||||||||||||
| crystal system | Monoclinic | ||||||||||||||
| Space group | P21/c | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Стабильность | Stable. | ||||||||||||||
| ИнЧИКей | KOCGCHBRHPOCBW-UHFFFAOYSA-N | ||||||||||||||
| Справочник по базе данных CAS | 1304-76-3(CAS DataBase Reference) | ||||||||||||||
| FDA UNII | A6I4E79QF1 | ||||||||||||||
| Справочник по химии NIST | bismuth(III) oxide(1304-76-3) | ||||||||||||||
| Система регистрации веществ EPA | Bismuth trioxide (1304-76-3) | ||||||||||||||
| UNSPSC Code | 12352303 | ||||||||||||||
| NACRES | NA.23 |
больше
| Коды опасности | Xi,Xn | |||||||||
| Заявления о рисках | 36/37/38-20/21/22 | |||||||||
| Заявления о безопасности | 26-36/37-36 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | EB2984460 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2825 90 85 | |||||||||
| Токсичность | LD50 orally in Rabbit: 5000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Висмута оксид химические свойства, назначение, производство
Химические свойства
Heavy, Yellow PowderФизические свойства
Yellow monoclinic crystal or powder; density 8.90 g/cm3; melts at 817°C; vaporizes at 1,890°C; insoluble in water; soluble in acids.Вхождение
Bismuth trioxide occurs in nature as mineral bismite. The oxide is used in fireproofing of papers and polymers; in enameling cast iron ceramic; and in disinfectants.Использование
Bismuth(III) oxide is used in the preparation of BiFeO3perovskite nanoparticles. It finds use in disinfectants, magnets, glass, rubber, vulcanization, fireproofing papers and polymers and catalysts. Bismuth trioxide brings about the "dragon's eggs" effect in fireworks, as a replacement of red lead. Bismuth(III) compounds are attractive reagents and catalysts in organic synthesis because of their low cost and ease of handling. Bismuth trioxide nanoparticles also play an important role in high energy gas generators. The alpha crystalline form of bismuth(III) oxide has p-type electronic conductivity.Подготовка
Bismuth trioxide is commercially made from bismuth subnitrate. The latter is produced by dissolving bismuth in hot nitric acid. Addition of excess sodium hydroxide followed by continuous heating of the mixture precipitates bismuth trioxide as a heavy yellow powder. Also, the trioxide can be prepared by ignition of bismuth hydroxide.Общее описание
Bismuth (III) oxide is a yellow, monoclinic crystalline powder. It is insoluble in water and hydroxide solutions but dissolves in acids to form bismuth (III) salts. It can be prepared by heating bismuth in air or by heating hydroxides, carbonates or nitrates of bismuth.Структура и конформация
Bismuth(III) Oxides are the deeply studied class of bismuth compounds, and they present four different phases. At room temperature, monoclinic α-Bi2O3 is the common stable phase with a polymeric-distorted layered structure composed of pentacoordinate bismuth atoms enclosed into pseudo-octahedral units. At a temperature higher than 710 °C, the α phase is converted into the cubic δ phase, which has a defective structure with random oxygen vacancies. The β phase and several oxygen-rich forms are closely related to the δ phase. In particular, the vacancy structures of highly defected bismuth oxides are some sites filled with O?2 and Bi(III) and Bi (V) sites. Bismuth oxide γ-phase also shows a cubic structure, but it is highly unstable and hard to synthesize without supporting it onto other oxides or metallic species. The other two polymorphic metastable bismuth oxide phases are known as the ω phase, stable at temperatures higher than 800 °C and the ε phase, isolated in 2006 by Cornei and co-workers[1].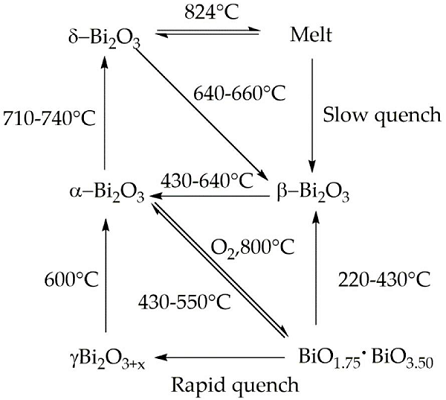
Висмута оксид запасные части и сырье
Висмута оксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-510-82753588 +86-13806194144 |
China | 300 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-027-59207850 | China | 5972 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +16837774622 | China | 295 | 58 | ||
| +86-17736087130 +86-18633844644 |
China | 994 | 58 |