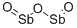
СУРЬМА оксид (III)
- английское имяDiantimony trioxide
- CAS №1309-64-4
- CBNumberCB3438204
- ФормулаO3Sb2
- мольный вес291.52
- EINECS215-175-0
- номер MDLMFCD00011214
- файл Mol1309-64-4.mol
| Температура плавления | 655 °C (lit.) | ||||||||||||||
| Температура кипения | 1550 °C (lit.) | ||||||||||||||
| плотность | 5.20 | ||||||||||||||
| Плотность накопления | 800-1300kg/m3 | ||||||||||||||
| давление пара | 13.3 hPa (660 °C) | ||||||||||||||
| Fp | 1550°C subl. | ||||||||||||||
| температура хранения | Store below +30°C. | ||||||||||||||
| растворимость | 2.70mg/l | ||||||||||||||
| форма | powder | ||||||||||||||
| Удельный вес | 5.67 | ||||||||||||||
| цвет | White | ||||||||||||||
| Запах | wh. cubic or orthorhombic cryst., odorless | ||||||||||||||
| Биологические источники | rabbit | ||||||||||||||
| Растворимость в воде | Slightly soluble. <0.1 g/100 mL at 20 ºC | ||||||||||||||
| Мерк | 14,711 | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Space group | Fd3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Пределы воздействия | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
||||||||||||||
| Стабильность | Stable. | ||||||||||||||
| ИнЧИКей | MUBFITUCTVFSOJ-UHFFFAOYSA-L | ||||||||||||||
| Справочник по базе данных CAS | 1309-64-4(CAS DataBase Reference) | ||||||||||||||
| Рейтинг продуктов питания EWG | 1 | ||||||||||||||
| FDA UNII | P217481X5E | ||||||||||||||
| МАИР | 2B (Vol. 47) 1989 | ||||||||||||||
| Справочник по химии NIST | Antimony trioxide(1309-64-4) | ||||||||||||||
| Система регистрации веществ EPA | Antimony trioxide (1309-64-4) | ||||||||||||||
| UNSPSC Code | 12352303 | ||||||||||||||
| NACRES | NA.41 |
| Коды опасности | Xn,Xi,T | |||||||||
| Заявления о рисках | 40-61 | |||||||||
| Заявления о безопасности | 22-36/37-45-53 | |||||||||
| РИДАДР | 1549 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | CC5650000 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28258000 | |||||||||
| Банк данных об опасных веществах | 1309-64-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: >20 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H360D:Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
СУРЬМА оксид (III) химические свойства, назначение, производство
Описание
This hard shiny metal is often alloyed to other elements. It is used in various industrial fields, such as those making or using batteries, printing machines, bearing, textiles, and ceramics. It caused positive patch test reactions in two workers in a ceramics industry.Химические свойства
White or gray mineral, sometimes pale red, white streak and adamantine or silky luster. Mohs hardness 2–3.Физические свойства
Occurs as colorless orthorhombic modifications, valentinite, or colorless cubic form, senarmontite; density 5.67 g/cm3 (valentinite), 5.20g/cm3 (senarmontite); cubic modification is dimeric consisting of Sb2O6 discrete molecules; refractive index 2.087; melts in the absence of oxygen at 656°C; boils at 1,550°C (sublimes); sublimes in vacuum at 400°C; very slightly soluble in water, insoluble in organic solvents; soluble in HCl, caustic alkalies and tartaric acid.Вхождение
Antimony trioxide occurs in nature as minerals, valentinite [1317-98-2] and senarmontinite [12412-52-1]. It is used as a flame retardant in fabrics; as an opacifier in ceramics, glass and vitreous enamels; as a catalyst; as a white pigment in paints; as a mortar in the manufacture of tartar emetic; and in the production of metallic antimony.Использование
manufacture of tartar emetic; as paint pigment; in enamels and glasses; as mordant; in flame-proofing canvas.Подготовка
Antimony trioxide is obtained by roasting stibnite:2 Sb2S3 + 9 O2 → 2Sb2O3 + 6SO2
Temperature and air feed is carefully controlled in the process to suppress any formation of antimony tetroxide (Sb2O4). Antimony trioxide is separated from any arsenic trioxide (As2O3) that may be present as an impurity by volatilization, as the latter is much more volatile than the former. It may be also prepared by alkaline hydrolysis of antimony trichloride and subsequent dehydration of hydrous oxide under controlled heating (rapid or vigorous heating may partially oxidize Sb(III) to Sb(V).
Antimony trioxide also may be made by heating the metallic element with oxygen or air. The volatilizing trioxide is condensed and collected.
Определение
A white insoluble solid. It is an amphoteric oxide with a strong tendency to act as a base. It can be prepared by direct oxidation by air, oxygen, or steam and is formed when antimony(III) chloride is hydrolyzed by excess boiling water.Общее описание
Diantimony trioxide is a white crystalline solid. Diantimony trioxide is insoluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Diantimony trioxide is used to fireproof fabrics, paper and plastics, as a paint pigment and for many other uses.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
IDiantimony trioxide ignites and burns when heated in powdered form in air [Mellor 9:425 1946-47]. Reacts violentlhy with bromine trifluoride [Mellor Vol. 9 425.1939].Опасность
Possible carcinogen during production.Угроза здоровью
DUST: POISONOUS IF INHALED OR IF SKIN IS EXPOSED. If inhaled will cause coughing, difficult breathing or loss of consciousness. SOLID: POISONOUS IF SWALLOWED OR IF SKIN IS EXPOSED. If swallowed will cause dizziness, nausea, vomiting or loss of consciousness.Пожароопасность
Not flammable.Контактные аллергены
This hard shiny metal is often alloyed to other elements. It is used in various industrial fields such as batteries, printing machines, bearing, textile, and ceramics. It caused positive patch test reactions in two workers in the ceramics industry.Профиль безопасности
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by intravenous and subcutaneous routes. Moderately toxic by other routes. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. See also ANTIMONY COMPOUNDS. When heated to decomposition it emits toxic Sb fumes. Incompatible with chlorinated rubber and heat of 21 6° and with BrF3.Возможный контакт
It is used in flame-proofing, pigments and ceramics, to stain iron and copper; to decolorize glass; industrial chemical, dye, pigment, and printing ink.Перевозки
UN1549 Antimony compounds, inorganic, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, halogenated acids, chlorinated rubber, bromine trifluoride. Reduction with hydrogen forms toxic antimony hydride.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.СУРЬМА оксид (III) запасные части и сырье
сырьё
1of3
запасной предмет
- polyester-based liquid crystalling ionmer containing sulfonate group
- Poly(butylene terephthalate)
- Сурьма
- Титана диоксид
- Sodium antimonate
1of3
СУРЬМА оксид (III) поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-86-1913198-3935 +8617331935328 |
China | 970 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 |
СУРЬМА оксид (III) Обзор)
1of4