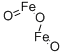
Железа (III) оксид
- английское имяFerric oxide
- CAS №1309-37-1
- CBNumberCB3115644
- ФормулаFe2O3
- мольный вес159.69
- EINECS215-168-2
- номер MDLMFCD00748135
- файл Mol1309-37-1.mol
| Температура плавления | 1538°C |
| плотность | 5.24 |
| Fp | >230 °F |
| температура хранения | 2-8°C |
| растворимость | It is soluble In Warm Hydrochloric Acid, Slightly Soluble in Sulfuric Acid. |
| форма | pieces |
| цвет | black |
| Удельный вес | 5.1~5.2 |
| РН | 3.7±0.3 |
| Растворимость в воде | INSOLUBLE |
| Мерк | 14,4028 |
| Пределы воздействия | ACGIH: TWA 5 mg/m3 OSHA: TWA 10 mg/m3; TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: IDLH 2500 mg/m3; TWA 5 mg/m3 |
| Стабильность | Stable. |
| Справочник по базе данных CAS | 1309-37-1(CAS DataBase Reference) |
| FDA 21 CFR | 186.1300; 73.1200; 73.200 |
| Вещества, добавляемые в пищу (ранее EAFUS) | FERRIC OXIDE |
| Специальный комитет по веществам GRAS | Ferric oxide Ferric oxide (packaging) |
| Рейтинг продуктов питания EWG | 1-2 |
| FDA UNII | 1K09F3G675 |
| МАИР | 3 (Vol. 1, Sup 7) 1987 |
| Справочник по химии NIST | Iron(iii) oxide(1309-37-1) |
| Система регистрации веществ EPA | Ferric oxide (1309-37-1) |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3 | |||||||||
| WGK Германия | - | |||||||||
| RTECS | NO7400000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28211000 | |||||||||
| Банк данных об опасных веществах | 1309-37-1(Hazardous Substances Data) | |||||||||
| ИДЛА | 2,500 mg Fe/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338+P310:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз. Немедленно обратиться за медицинской помощью.
Железа (III) оксид химические свойства, назначение, производство
Описание
Iron oxides are produced synthetically and consist essentially of anhydrous and/or hydrated iron oxides. The range of hues includesyellows, reds, browns and blacks. Food quality iron oxides are primarily distinguished from technical grades by the comparatively low levels of contamination by other metals. This is achieved by the selection and control of the source of iron and/or by the extent of chemical purification during the manufacturing process. Iron oxides have been used to color confectionery, fillings and decorations for pastry products, cheese products, fish paste, pet foods, cosmetics and pharmaceutical products.Химические свойства
Hematite is a noncombustible, black to black red or brick-red mineral (iron ore) composed mainly of ferric oxide, Fe2O3. Ferric oxideВхождение
Iron(III) oxide occurs in nature as the mineral hematite. It is the principal ore of iron from which the metal and its alloys are produced. Also, this oxide occurs in the mineral, limonite, 2Fe2O3?3H2O. An important application of this compound involves producing red, orange, and yellow pigments. Other applications are in coatings for metals, steel and rubber; in ceramics; and as a catalyst for oxidation reactions.Использование
Ferric Oxide is a nutrient and dietary supplement that is a source of iron.Подготовка
Iron(III) oxide is prepared as a reddish-brown hydrated precipitate by treating an aqueous solution of an iron(III) salt with caustic soda:2FeCl3 + 6NaOH → Fe2O3?3H2O + 6NaCl
It also is obtained by thermal decomposition of iron(II) sulfate or the brown oxide hydroxide:
2FeSO4 → Fe2O3 + SO2 + SO3
2FeO(OH) → Fe2O3 + H2O
The oxide is prepared in industrial scale by first precipitating iron(II) hydroxide Fe(OH)2 by treating aqueous solutions of iron(II) sulfate and caustic soda. The Fe(OH)2 is then oxidized to iron(III) hydroxide by aeration. The latter is dehydrated by heating:
Fe2+ (aq) + OHˉ (aq) → Fe(OH)2(s) → 2Fe(OH)3 → Fe2O3 + 3H2O
It also is produced by ignition of iron(III) oxalate and iron carbonyls:
2Fe2(C2O4)3 +3O2 → 2Fe2O3 + 12CO
.
Определение
A high-grade red pigment used as a polishing agent for glass, jewelry, etc. (2) A cosmetic prepared from dried flowers of the saf- flower.Опасность
Pneumoconiosis. Questionable carcinogen.Возможный контакт
Hematite; as an iron ore composed mainly of ferric oxide, is a major source of iron and is used as a pigment for rubber, paints, paper, linoleum, ceramics, dental restoratives; and as a polishing agent for glass and pre cious metals. It is also used in electrical resistors, semiconduc tors, magnets, and as a catalyst. Human exposure to hematite from underground hematite mining is principally through inhalation and/or ingestion of dusts. No estimates are available concerning the number of underground miners exposed.Канцерогенность
Welders are typically exposed to a complex mixture of dust and fume of metallic oxides, as well as irritant gases, and are subject to mixeddust pneumoconiosis with possible loss of pulmonary function; this should not be confused with benign pneumoconiosis caused by iron oxide.1 Although an increased incidence of lung cancer has been observed among hematite miners exposed to iron oxide, presumably owing to concomitant radon gas exposure, there is no evidence that iron oxide alone is carcinogenic to man or animals.6Несовместимости
Contact with hydrogen peroxide, ethyl ene oxide, calcium hypochlorite will cause explosion. Violent reaction with powdered aluminum; hydrazine, hydrogen trisulfide.Железа (III) оксид запасные части и сырье
сырьё
1of5
запасной предмет
1of2
Железа (III) оксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 0086 15632927689 +8615632927689 |
China | 984 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2503 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 2989 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 970 | 58 | |
| +8613343047651 | China | 3002 | 58 | |
| +8615373025980 | China | 870 | 58 | |
| +86-0531-8875-2665 +8613153039501 |
China | 531 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21670 | 55 | |
| 008657128800458; +8615858145714 |
China | 9337 | 55 | |
| 18017610038 | CHINA | 3620 | 58 |