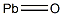
Свинец(II) оксид
- английское имяLead monoxide
- CAS №1317-36-8
- CBNumberCB4119629
- ФормулаOPb
- мольный вес223.1994
- EINECS215-267-0
- номер MDLMFCD00011164
- файл Mol1317-36-8.mol
| Температура плавления | 886 °C(lit.) | ||||||||||||||
| Температура кипения | 1470 °C | ||||||||||||||
| плотность | 9.53 | ||||||||||||||
| Плотность накопления | 3500-3700kg/m3 | ||||||||||||||
| давление пара | 10 mm Hg ( 0 °C) | ||||||||||||||
| показатель преломления | 2.67 | ||||||||||||||
| температура хранения | Store below +30°C. | ||||||||||||||
| растворимость | Soluble in concentrated alkali, HCl and ammonium chloride. Insoluble in dilute alkali and alcohol. | ||||||||||||||
| форма | powder | ||||||||||||||
| цвет | yellow | ||||||||||||||
| Удельный вес | 9.53 | ||||||||||||||
| РН | 8-9 (100g/l, H2O, 20℃)(slurry) | ||||||||||||||
| Растворимость в воде | Soluble in concentrated alkali, hydrochloric acid, and ammonium chloride. Insoluble in water, dilute alkali and alcohol. | ||||||||||||||
| Кристальная структура | Rutile type | ||||||||||||||
| Гидролитическая чувствительность | 4: no reaction with water under neutral conditions | ||||||||||||||
| Мерк | 14,5413 | ||||||||||||||
| crystal system | square | ||||||||||||||
| Space group | P42/mnm | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Пределы воздействия | ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
||||||||||||||
| Стабильность | Stable. Reacts violently with hydrogen peroxide, strong oxidizing agents, aluminium, zirconium, halogens, sulphur trioxide, boron, silicon, sodium, zinc. | ||||||||||||||
| Справочник по базе данных CAS | 1317-36-8(CAS DataBase Reference) | ||||||||||||||
| Рейтинг продуктов питания EWG | 2 | ||||||||||||||
| FDA UNII | 4IN6FN8492 | ||||||||||||||
| Справочник по химии NIST | Lead monoxide(1317-36-8) | ||||||||||||||
| Система регистрации веществ EPA | Lead monoxide (1317-36-8) | ||||||||||||||
| UNSPSC Code | 12352303 | ||||||||||||||
| NACRES | NB.24 |
| Коды опасности | T,N | |||||||||
| Заявления о рисках | 61-20/22-33-50/53-62 | |||||||||
| Заявления о безопасности | 53-45-60-61 | |||||||||
| РИДАДР | UN 2291 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | OG1750000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2824 10 00 | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 1317-36-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 i.p. in rats: 40 mg Pb/100g (Bradley, Fredrick) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H372:Поражает органы в результате многократного или продолжительного воздействия.
H302+H332:Вредно при проглатывании или при вдыхании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H360D:Может отрицательно повлиять на неродившегося ребенка.
H362:Может причинить вред детям, находящимся на грудном вскармливании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P263:Избегать контакта в период беременности и грудного вскармливания.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Свинец(II) оксид химические свойства, назначение, производство
Описание
Lead(II) oxide occurs in two polymorphs, red, having a tetragonal crystal structure and yellow, having an orthorhombic crystal structure. Both forms occur naturally as rare minerals. The red form is known as “Litharge” and the yellow form is known as “Massico”.Химические свойства
Lead monoxide, litharge, PbO, exists in a reddish alpha form up to 489 °C; it then transforms to a yellow beta form (massicot), which is stable at high temperatures. It has a water solubility of 17 mg/L at 20 °C, and is soluble in nitric acid, alkalies, lead acetate, ammonium chloride, and chlorides of calcium and strontium. In alkalies, it forms the plumbite ion, [PbO2]2? . Lead oxides are produced industrially by thermal processes in which lead is directly oxidized with air. In the ball mill process, metallic lead balls are tumbled in air to produce a “leady” oxide, which typically contains 20-35% free lead. The Barton pot process oxidizes droplets of molten lead at ca. 430°C to produce either litharge or leady litharge.Физические свойства
The oxide exhibits two crystalline modifications, the reddish or orange-red alpha form, known as litharge, and the yellow beta form, massicot. The alpha form constitutes tetragonal crystals while the beta modification is a yellow amorphous powder of orthorhombic crystal structure. The alpha form is stable at ordinary temperatures, converting to the beta form when heated at 489°C; density 9.35 g/cm3 (beta form); Moh’s hardness 2 (alpha form); the oxide melts at 888°C; vaporizes at 1,472°C with decomposition; vapor pressure 1 torr at 943°C and 5 torr at 1,039°C; practically insoluble in water (the solubility of alpha form is 17 mg/L at 20°C and that of beta form 23 mg/L at 22°C); insoluble in ethanol; soluble in dilute nitric acid and alkalies.Использование
Lead(II) oxide is employed mostly in lead-based industrial glass and industrial ceramics, including computer components. It is used as an intermediate/precursor in the manufacture of several products, for example water proof cements, lubricants, lubricating oils, inorganic pigments, lead soaps, petroleum refining, rubber, cathode ray tube glass, and polyvinyl chloride (PVC). It is useful for lead acid batteries as cathode and anode. Lead monoxide scores significant applications in oil, gas and chemical manufactures. It is an efficient catalyst for condensation reactions in organic synthesis.Общее описание
Odorless gray or yellow green or red-brown solid. Sinks in water.Профиль реактивности
Lead monoxide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. Aluminum carbide is oxidized with incandescence on warming with lead oxide, [Mellor, 1946, Vol. 5, 872]. Mixtures of lead oxide with aluminum powder(as with other metals: sodium, zirconium) give a violent explosions, [Mellor, 1946, Vol. 5, 217, 1941].Угроза здоровью
General symptoms of lead poisoning (delayed). Inhalation or ingestion causes abdominal pain (lead colic), metallic taste in mouth, loss of weight, pain in muscles, and muscular weakness. Dust may irritate eyes.Методы очистки
Higher oxides are removed by heating under vacuum at 550o with subsequent cooling under vacuum. It is red at room temperature but becomes yellow at high temperatures (~480o) reversibly. [Ray & Ogg J Am Chem Soc 78 5994 1956, Kwestroo et al. J Inorg Nucl Chem 29 39 1967.]Свинец(II) оксид запасные части и сырье
сырьё
запасной предмет
- LEAD (II) BORATE MONOHYDRATE
- ХРОМАТ СВИНЦА
- Lead sulfate tribasic
- Lead isooctanoate
- LEAD PHOSPHITE, DIBASIC
- Свинец(II) ацетат основной
- Various color amino baking enamel A04-9
- Lead(II) tetrafluoroborate
- LEAD SILICOCHROMATE
- Dibasic Lead Phthalate
1of6
Свинец(II) оксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | |
| +86-13131129325 | China | 5871 | 58 | |
| +8617736087130 | China | 994 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21630 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29861 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8617531153977 | China | 5854 | 58 | |
| 13180553332 | CHINA | 990 | 58 | |
| +8613367258412 | China | 10244 | 58 |
Свинец(II) оксид Обзор)
1of4