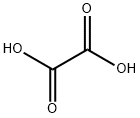
Щавелевая кислота
- английское имяOxalic acid
- CAS №144-62-7
- CBNumberCB0323998
- ФормулаC2H2O4
- мольный вес90.03
- EINECS205-634-3
- номер MDLMFCD00002573
- файл Mol144-62-7.mol
| Температура плавления | 189.5 °C (dec.)(lit.) |
| Температура кипения | 365.1°C (estimate) |
| плотность | 0.99 g/mL at 25 °C |
| Плотность накопления | 750kg/m3 |
| плотность пара | 4.4 (vs air) |
| давление пара | <0.01 mm Hg ( 20 °C) |
| показатель преломления | 1.4261 (estimate) |
| Fp | 101-157°C |
| температура хранения | Store below +30°C. |
| растворимость | water: soluble108g/L at 25°C |
| форма | Liquid |
| пка | 1.23(at 25℃) |
| цвет | White |
| Водородный показатель | 6 - 8 at 25 °C |
| РН | 3(1 mM solution);2.09(10 mM solution);1.31(100 mM solution); |
| Запах | odorless |
| Растворимость в воде | 90 g/L (20 ºC) |
| Сублимация | 101-157 ºC |
| Мерк | 14,6911 |
| БРН | 385686 |
| констант закона Генри | 1.43 at pH 4 (quoted, Gaffney et al., 1987) |
| Пределы воздействия | NIOSH REL: TWA 1, STEL 2, IDLH 500; OSHA PEL: TWA 1; ACGIH TLV: TWA 1, STEL 2 (adopted). |
| Стабильность | Stable, but moisture sensitive. Incompatible with metals. |
| ИнЧИКей | MUBZPKHOEPUJKR-UHFFFAOYSA-N |
| LogP | -1.7 at 23℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | OXALIC ACID |
| FDA 21 CFR | 177.2410 |
| Справочник по базе данных CAS | 144-62-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2-4 |
| FDA UNII | 9E7R5L6H31 |
| Справочник по химии NIST | Oxalic acid(144-62-7) |
| Система регистрации веществ EPA | Oxalic acid (144-62-7) |
| UNSPSC Code | 41116107 |
| NACRES | NB.21 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 21/22-63-34-41 | |||||||||
| Заявления о безопасности | 24/25-23-36/37/39-27-26-39-37-36-36/37 | |||||||||
| РИДАДР | UN 3261 8/PG 3 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 1 mg/m3, STEL: 2 mg/m3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | RO2450000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29171110 | |||||||||
| Банк данных об опасных веществах | 144-62-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 375 mg/kg | |||||||||
| ИДЛА | 500 mg/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
H302+H312:Вредно при проглатывании или при попадании на кожу.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Щавелевая кислота химические свойства, назначение, производство
Химические свойства
Oxalic acid is a colorless, odorless powder, or granular solid. The anhydrous form (COOH)2 is an odorless, white solid; the solution is a colorless liquid.Физические свойства
Colorless and odorless rhombic crystals. Hygroscopic. soluble in ethanol, soluble in water, slightly soluble in ether, insoluble in benzene and chloroform.Использование
Oxalic acid was used: · in the synthesis of hemicellulose hydrolysates of yellow poplars; · in the synthesis of three-dimensionally ordered macroporous metal oxides or carbonates via templating with polystyrene spheres; · as supporting electrolyte in the electrochemical synthesis of polyaniline-polypyrrole composite coatings.Методы производства
Many industrial processes have been employed for the manufacture of oxalic acid since it was first synthesized. The following processes are in use worldwide: oxidation of carbohydrates, the ethylene glycol process, the propylene process, the dialkyl oxalate process, and the sodium formate process. Sodium formate process is no longer economical in the leading industrial countries, except for China.Nitric acid oxidation is used where carbohydrates, ethylene glycol, and propylene are the starting materials. The dialkyl oxalate process is the newest, where dialkyl oxalate is synthesized from carbon monoxide and alcohol, then hydrolyzed to oxalic acid. This process has been developed by UBE Industries in Japan.Many attempts have been made to synthesize oxalic acid by electrochemical reduction of carbon dioxide in either aqueous or nonaqueous electrolytes.
Определение
ChEBI: Oxalic acid is an alpha,omega-dicarboxylic acid that is ethane substituted by carboxyl groups at positions 1 and 2. It has a role as a human metabolite, a plant metabolite and an algal metabolite. It is a conjugate acid of an oxalate(1-) and an oxalate.Реакции
The reactions of oxalic acid, including the formation of normal and acid salts and esters, are typical of the dicarboxylic acids class. Oxalic acid, however, does not form an anhydride.On rapid heating, oxalic acid decomposes to formic acid, carbon monoxide, carbon dioxide, and water. In aqueous solution, it is decomposed by uv, x-ray, or γ -radiation with the liberation of carbon dioxide. Photodecomposition also occurs in the presence of uranyl salts.
Oxalic acid is a mild reducing agent, and is oxidized by potassium permanganate in acid solution to give carbon dioxide and water. Oxalic acid is catalytically reduced by hydrogen in the presence of ruthenium catalyst to ethylene glycol, and electronically reduced to glyoxylic acid.
Oxalic acid reacts with various metals to form metal salts, which are quite important as the derivatives of oxalic acid. It also reacts easily with alcohols to give esters.
Общее описание
Odorless white solid. Sinks and mixes with water.Реакции воздуха и воды
Water soluble. HygroscopicПрофиль реактивности
Oxalic acid is hygroscopic and sensitive to heat. Oxalic acid may react violently with furfuryl alcohol, silver, sodium, perchlorate, sodium hypochlorite, strong oxidizers, sodium chlorite, acid chlorides, metals and alkali metals. . The heating of mixtures of Oxalic acid and urea has lead to explosions. This is due to the rapid generation of the gases, CO2, CO, and NH3, [Praxis Naturwiss. Chem., 1987, 36(8), 41-42]. Oxalic acid and urea react at high temperatures to form toxic and flammable ammonia and carbon monoxide gasses, and inert CO2 gas [Von Bentzinger, R. et al., Praxis Naturwiss. Chem., 1987, 36(8), 41-42].Угроза здоровью
As dust or as a solution, can cause severe burns of eyes, skin, or mucous membranes. Ingestion of 5 grams has caused death with symptoms of nausea, shock, collapse, and convulsions coming on rapidly. Repeated or prolonged skin exposure can cause dermatitis and slow-healing ulcers.Пожароопасность
Special Hazards of Combustion Products: Generates poisonous gasesСельскохозяйственное использование
Oxalic acid, (COOH)2, also called ethanedioic acid, is a white, crystalline solid, slightly soluble in water. It is a naturally occurring highly oxidized organic compound with significant chelating activity. It is strongly acidic and poisonous, produced by many plants like sorrel (sourwood), the leaf blades of rhubarb, bark of eucalyptus and many plant roots. In plant cells and tissues, oxalic acid gets accumulated as either sodium, potassium or calcium oxalate, of which the latter occurs as crystals. In turn, salts of oxalic acids enter the bodies of animals and human beings, causing pathological disorders, depending upon the amount consumed. Many species of fungi like Aspergillus, Penicillium, Mucor, as well as some lichens and slime moulds produce calcium oxalate crystals. Upon the death of these microorganisms, plants and animals, the salts get released into the soil, causing some amount of toxicity. However, oxalate-degrading microbes, called Oxalobacter formigenes, decrease oxalate absorption in animals and humans.Oxalic acid is the first of a series of dicarboxylic acids. It is used (a) as a bleaching agent for stains like rust or ink, (b) in textile and leather production, and (c) as monoglyceryl oxalate in the production of ally1 alcohol and formic acid.
Возможный контакт
Oxalic acid is used in textile finishing, paint stripping; metal and equipment cleaning; as an intermediate; as an analytic reagent and in the manufacture of dyes, inks, bleaches, and paint removers; varnishes, wood, and metal cleansers; dextrin, cream of tartar, celluloid, oxalates, tartaric acid, purified methyl alcohol, glycerol, and stable hydrogen cyanide. It is also used in the photographic, ceramic, metallurgic, rubber, leather, engraving, pharmaceutical, paper, and lithographic industries.Экологическая судьба
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 0.12 g/g which is 66.7% of the ThOD value of 0.18 g/g.Chemical/Physical. At temperatures greater than 189.5 °C, decomposes to carbon dioxide, carbon monoxide, formic acid, and water (Windholz et al., 1983). Ozonolysis of oxalic acid in distilled water at 25 °C under acidic conditions (pH 6.3) yielded carbon dioxide (Kuo et al., 1977). Absorbs moisture in air forming the dihydrate (Huntress and Mulliken, 1941).
Reacts with bases forming water soluble salts.
Перевозки
UN3261 Corrosive solid, acidic, organic, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.Несовместимости
The aqueous solution is a medium-strong acid. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from silver compounds; strong alkalis; chlorites. Contact with some silver compounds forms explosive materials.Утилизация отходов
Pretreatment involves chemical reaction with limestone or calcium oxide forming calcium oxalate. This may then be incinerated utilizing particulate collection equipment to collect calcium oxide for recycling.Щавелевая кислота запасные части и сырье
сырьё
1of3
запасной предмет
- 7 - (трифторметил) хинолин
- щавелевая кислота, калиевая соль
- Розин
- Potassium binoxalate
- Ferric ammonium oxalate
- оксалат никеля(II)
- Lincomycin hydrochloride monohydrate
- Натрий оксалат
- ОКСАЛАТ АММОНИЯ
- alpha-(1-Naphthylmethyl)-2-tetrahydrofuranpropionic acid diethylaminoethyl ester oxalate
1of6
Щавелевая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +1-+1(833)-552-7181 | United States | 57505 | 58 | ||
| +8613393234347 | China | 2946 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +8615689548120 | China | 193 | 58 |