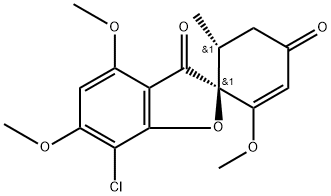
Гризеофульвин
- английское имя(+)-Griseofulvin
- CAS №126-07-8
- CBNumberCB1766075
- ФормулаC17H17ClO6
- мольный вес352.77
- EINECS204-767-4
- номер MDLMFCD00082343
- файл Mol126-07-8.mol
| Температура плавления | 218-220 °C(lit.) |
| Температура кипения | 469.04°C (rough estimate) |
| альфа | 354 º (c=1, dimethylform) |
| плотность | 1.2579 (rough estimate) |
| показатель преломления | 1.4429 (estimate) |
| температура хранения | 2-8°C |
| растворимость | Practically insoluble in water, freely soluble in dimethylformamide and in tetrachloroethane, slightly soluble in anhydrous ethanol and in methanol |
| форма | Powder |
| цвет | White to yellow-white |
| Растворимость в воде | practically insoluble |
| λмакс | 321nm(CHCl3)(lit.) |
| Мерк | 14,4549 |
| БРН | 95226 |
| BCS Class | 2 (CLogP), 4 (LogP) |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO, ethanol, or DMF may be stored at -20° for up to 3 months. |
| ИнЧИКей | DDUHZTYCFQRHIY-RBHXEPJQSA-N |
| LogP | 2.180 |
| Справочник по базе данных CAS | 126-07-8(CAS DataBase Reference) |
| FDA UNII | 32HRV3E3D5 |
| Справочник по химии NIST | Griseofulvin(126-07-8) |
| Предложение 65 Список | Griseofulvin |
| Код УВД | D01AA08,D01BA01 |
| МАИР | 2B (Vol. Sup 7, 79) 2001 |
| Система регистрации веществ EPA | Griseofulvin (126-07-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T,Xn | |||||||||
| Заявления о рисках | 60-61-40-43-45 | |||||||||
| Заявления о безопасности | 53-22-36/37/39-45 | |||||||||
| РИДАДР | UN 1648 3 / PGII | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | WG9800000 | |||||||||
| кода HS | 29419090 | |||||||||
| Банк данных об опасных веществах | 126-07-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral in rat: > 10gm/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Гризеофульвин химические свойства, назначение, производство
Химические свойства
Crystalline SolidИспользование
It is an antifungal drug. It is used both in animal and in humans, to treat rigworm infections of the skin and nails. It is derived from the mold Penicillium griseofulvum.Environmental contaminants; Food contaminants.Определение
ChEBI: An oxaspiro compound produced by Penicillium griseofulvum. It is used by mouth as an antifungal drug for infections involving the scalp, hair, nails and skin that do not respond to topical treatment.Показания
Griseofulvin (Fulvicin, Grifulvin V) has been used safely and effectively for decades for dermatophyte infections of scalp and nails and for more widespread skin eruptions. However, infections in certain sites (e.g.. toenails) respond poorly. The drug is generally well tolerated, even in the long-term courses necessary for nail disease.Всемирная организация здравоохранения(ВОЗ)
Griseofulvin, isolated from a penicillin producing mould, has been widely used as a systemically administered antifungal agent in man for over 20 years. It is effective in dermatophyte infections (including tinea barbae and tinea capitis) but it is inactive against yeasts and bacteria. Evidence that very high doses of griseofulvin are carcinogenic, teratogenic and fetotoxic in laboratory animals has led to an acceptance that it should not be used to treat trivial infections that respond to topical therapy. Oral formulations of griseofulvin are included in the WHO Model List of Essential Drugs. (Reference: (WHTAC1) The Use of Essential Drugs, 2nd Report of the WHO Expert Committee, 722, , 1985)Антимикробная активность
The spectrum of useful activity is restricted to dermatophytes causing skin, nail and hair infections (Epidermophyton, Microsporum and Trichophyton spp.). Resistance has seldom been reported.Общее описание
White to pale cream-colored crystalline powder. Odorless or almost odorless. Tasteless. Sublimes without decomposition at 410°F.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
(+)-Griseofulvin is incompatible with strong oxidizing agents. .Опасность
Possible carcinogen.Пожароопасность
Flash point data for (+)-Griseofulvin are not available. (+)-Griseofulvin is probably combustible.Фармацевтические приложения
A fermentation product of various species of Penicillium, including Pen. griseofulvum. Available as fine-particle or ultrafine- particle formulations for oral use.Механизм действия
The mechanism of action of griseofulvin is through binding to the protein tubulin, which interferes with the function of the mitotic spindle and, thereby, inhibits cell division. Griseofulvin also may interfere directly with DNA replication. Griseofulvin is gradually being replaced by newer agents .Фармакокине?тика
Absorption from the gastrointestinal tract is dependent on drug formulation. Administration with a high-fat meal will increase the rate and extent of absorption, but individuals tend to achieve consistently high or low blood concentrations. It appears in the stratum corneum within 4–8 h as a result of secretion in perspiration. However, levels begin to fall soon after the drug is discontinued, and within 48–72 h it can no longer be detected. It is metabolized in the liver, the metabolites being excreted in the urine. The elimination half-life is 9–21 h.Клиническое использование
Dermatophyte infections of hair, skin and nailПобочные эффекты
Adverse reactions occur in about 15% of patients and include headache, nausea, vomiting, rashes and photosensitivity.Метаболизм
Griseofulvin is metabolized by the liver, and its metabolites are excreted in the urine. It is highly protein bound and, therefore, may have high tissue levels. However, tissue levels decrease and fall as soon as griseofulvin is discontinued such that its presence is no longer detectable 2 to 3 days after cessation of therapy.Методы очистки
Crystallise it from *benzene or EtOH. Purify 2g of griseofulvin by chromatography on Alumina (40 x 1.5cm) and elute with *C6H6/MeOH (199:1) and follow the UV blue fluorescent band. [MacMillan J Chem Soc 1823 1959, Beilstein 18 III/IV 3160, 18/5 V 150.]Гризеофульвин запасные части и сырье
Гризеофульвин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618523575427 | China | 49732 | 58 | ||
| +8613367258412 | China | 10319 | 58 | ||
| +86-13591747876 +86-13591747876 |
China | 299 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-027-59207850 | China | 5972 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +8618092446649 | China | 1143 | 58 |