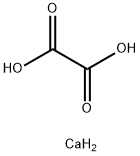
Оксалат кальция
- английское имяCalcium oxalate
- CAS №563-72-4
- CBNumberCB7349915
- ФормулаC2H4CaO4
- мольный вес132.13
- EINECS209-260-1
- номер MDLMFCD00012474
- файл Mol563-72-4.mol
| Температура плавления | decomposes [CRC10] |
| плотность | 2.2 g/mL at 25 °C (lit.) |
| форма | Powder |
| цвет | White |
| Растворимость в воде | g/L solution H2O: 0.0069 (25°C), 0.0142 (95°C); solid phase, CaC2O4 ·H2O [KRU93]; soluble dilute HCl, HNO3 [HAW93] |
| Мерк | 13,1690 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| Справочник по базе данных CAS | 563-72-4(CAS DataBase Reference) |
| FDA UNII | 2612HC57YE |
| Система регистрации веществ EPA | Ethanedioic acid, calcium salt (1:1) (563-72-4) |
| UNSPSC Code | 12352302 |
| Коды опасности | Xn |
| Заявления о рисках | 20/21/22-36/37/38-21/22 |
| Заявления о безопасности | 26-37/39-24/25 |
| WGK Германия | 3 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302+H312:Вредно при проглатывании или при попадании на кожу.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P362+P364:Снять всю загрязненную одежду и выстирать ее перед повторным использованием.
Оксалат кальция химические свойства, назначение, производство
Описание
Calcium oxalate (in archaic terminology, oxalate of lime) is a chemical compound that forms envelope-shaped crystals, known in plants as raphides. A major constituent of human kidney stones, the chemical is also found in beerstone, a scale that forms on containers used in breweries. Its chemical formula is CaC2O4 or Ca (COO)2.Химические свойства
Calcium oxalate is white precipitate, insoluble in weak acids, but soluble in strong acids, formed by reaction of soluble calcium salt solution and ammonium oxalate solution. Solubility at 18 °C 0.0056 g anhydrous salt per liter of saturated solution.Физические свойства
Most crystals look like a 6 sided prism and often look like a pointed picket from a wooden fence. More than 90 % of the crystals in a urine sediment will have this type of morphology. These other shapes are less common than the 6 sided prism, however it is important to be able to quickly identify them in case of emergency.Вхождение
Many plants are accumulating calcium oxalate (it has been reported in 1000 genera of tree ). The calcium oxalate accumulation is linked to the detoxification of calcium (Ca2+) in the plant.Calcium oxalate is a poisonous substance that can produce sores and numbing on ingestion and could even be fatal.
The poisonous plant dumb cane (Dieffenbachia) contains the substance and on ingestion can prevent speech and be suffocating. It is also found in rhubarb (in large quantities in the leaves) and in species of Oxalis, Araceae, taro, kiwifruit, tea leaves, agaves, and Alocasia and in spinach in varying amounts. Insoluble calcium oxalate crystals are found in plant stems, roots, and leaves and produced in idioblasts. Calcium oxalate, as ' beer stone ', is a brownish precipitate that tends to accumulate within vats, barrels and other containers used in the brewing of beer.
прикладной
Calcium oxalate is used in the manufacture of ceramic glazes.Определение
ChEBI: The calcium salt of oxalic acid, which in excess in the urine may lead to formation of oxalate calculi (kidney stones).Общее описание
Purity based on trace metal analysisУгроза здоровью
Even a small dose of calcium oxalate is enough to cause intense sensations of burning in the mouth and throat, swelling, and choking that could last for up to two weeks . In greater doses it can cause severe digestive upset, breathing difficulties, coma or even death. Recovery from severe oxalate poisoning is possible, but permanent liver and kidney damage may have occurred.The stalks of plants in the Dieffenbachia genus produce the most severe oxalate reactions. The needle - like oxalate crystals produce pain and swelling when they contact lips, tongue, oral mucosa, conjunctiva, or skin. Edema primarily is due to direct trauma from the needle-like crystals and, to a lesser extent, by other plant toxins (e.g., bradykinins, enzymes).
Depending on the plant ingested, mild (Elephant Ear Colocasia esculenta) to more severe (Jack in the Pulpit, Arisaema) can cause compromised airways. One bite on the Arisaema seed pod will result in immediate swelling and burning. It will take over 12 hours for the swelling to subside .
4 – 1 - Treatment
Medication administered at the emergency room may include diphenhydramine, epinephrine, or famotidine, all intravenously. Although this most likely will be a localized reaction, it will be treated by the ER as an anaphylactic reaction.
Оксалат кальция запасные части и сырье
Оксалат кальция поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +8613073028829 | China | 2994 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 |