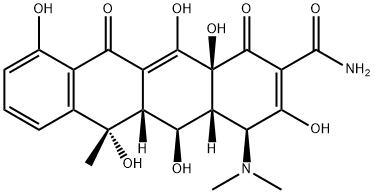
Окситетрациклин
- английское имяOxytetracycline
- CAS №79-57-2
- CBNumberCB6429340
- ФормулаC22H24N2O9
- мольный вес460.43
- EINECS201-212-8
- номер MDLMFCD00003700
- файл Mol79-57-2.mol
| Температура плавления | 183 °C |
| альфа | -223 º (c=1 0.03N HCl) |
| Температура кипения | 565.29°C (rough estimate) |
| плотность | 1.6340 |
| показатель преломления | 1.6500 (estimate) |
| температура хранения | Keep in dark place,Inert atmosphere,Room temperature |
| растворимость | Sparingly soluble in aqueous solution at 0.6 mg/mL |
| пка | pKa 3.27/7.32/9.11(H2O,t =25,I<0.01)(Approximate) |
| форма | Crystalline Powder |
| цвет | Beige to light yellow |
| Биологические источники | rabbit |
| Растворимость в воде | 0.2 g/L |
| ИнЧИКей | IWVCMVBTMGNXQD-PXOLEDIWSA-N |
| LogP | -0.900 |
| FDA 21 CFR | 556.500; 558.450; 558.4 |
| Справочник по базе данных CAS | 79-57-2(CAS DataBase Reference) |
| FDA UNII | SLF0D9077S |
| Предложение 65 Список | Oxytetracycline (internal use) |
| Код УВД | D06AA03,G01AA07,J01AA06,J01AA56,S01AA04 |
| Пестициды Закон о свободе информации (FOIA) | Oxytetracycline |
| Система регистрации веществ EPA | Oxytetracycline (79-57-2) |
| UNSPSC Code | 12352203 |
| NACRES | NA.41 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 63-36-36/37/38 | |||||||||
| Заявления о безопасности | 22-24/25-36/37/39-26-36 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | QI7875000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 30029090 | |||||||||
| Банк данных об опасных веществах | 79-57-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral in rat: 4800mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H411:Токсично для водных организмов с долгосрочными последствиями.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P391:Ликвидировать просыпания/проливы/утечки.
Окситетрациклин химические свойства, назначение, производство
Описание
Oxytetracycline also is one of the classic tetracyclines. It is produced by fermentation of Streptomyces rimosis and other soil microorganisms. The most hydrophilic tetracycline on the market, it has largely now been replaced by its semisynthetic descendants. It is primarily used today for IM injections.Химические свойства
beige to light yellow crystalline powderИспользование
Oxytetracycline(Terramycin) was the second of the broad-spectrum tetracycline group of antibiotics to be discovered. Oxytetracycline works by interfering with the ability of bacteria to produce proteins that are essential to them. Without these proteins tОпределение
ChEBI: A tetracycline used for treatment of infections caused by a variety of Gram positive and Gram negative microorganisms including Mycoplasma pneumoniae, Pasteurella pestis, Escherichia coli, Haemophilus influenzae /ital> (respiratory infections), and Diplococcus pneumoniae.Антимикробная активность
It is slightly less active than other tetracyclines against most common pathogenic bacteria.Фармацевтические приложения
A fermentation product of certain strains of Streptomyces rimosus, supplied as the dihydrate or hydrochloride for oral or parenteral administration.Фармакокине?тика
Oral absorption: c.60%Cmax 500 mg oral: 3–4 mg/L after 2–4 h
Plasma half-life: c.9 h
Volume of distribution: c.1.8 L/kg
Plasma protein binding: 20–35%
Oxytetracycline is moderately well absorbed from the upper gastrointestinal tract. Food decreases plasma levels by approximately 50%. Although widely distributed in the tissues, it achieves lower concentrations than related agents such as minocycline. Sputum concentrations of 1 mg/L have been recorded on a daily dosage of 2 g. Approximately 60% is excreted in the urine and the half-life is prolonged in renal insufficiency.
Клиническое использование
It offers no unique therapeutic advantages, although it is one of the cheaper preparations.Побочные эффекты
Gastrointestinal intolerance is responsible for most side effects, and tends to be more severe than with other tetracyclines. Esophageal irritation may result from the local effects of the swallowed drug. Potentially serious adverse reactions have included neuromuscular paralysis following intravenous administration to patients with myasthenia gravis. Thrombocytopenic purpura and lupus erythematosus syndrome have been reported, although a direct role for the drug in the latter remains uncertain. Apart from the effect on nitrogen balance common to many tetracyclines, a metabolic effect on glucose homeostasis has been noted in type 1 diabetes mellitus. Allergic contact sensitivity reactions have also been reported.Методы очистки
Terramycin crystallises (as dihydrate) from water or aqueous EtOH and is soluble in MeOH, EtOH, Me2CO and H2O (0.25mg/mL at 25o ) but insoluble in Et2O and pet ether. It is amphoteric, and an aqueous solution has pH 2.0-5.0. It has max at 247, 275 and 353nm in 0.1 M phosphate (pH 4.5). [Finlay et al. Science 111 85 1951.] The hydrochloride, [2058-46-0] M 496.9 [Beilstein 14 IV 2630], crystallises from MeOH in needles and from H2O at 50o it forms plates. Terramycin has also been purified via the hydrochloride by dissolving it in H2O, adjusting to pH 6, and the solid is filtered off after 1hour. The crystals of the dihydrate are dried to constant weight in vacuum/CaCl2/25o. Drying at 60o in vacuo gives the anhydrous base m 184.5-185.5o (sintering at 180o). The dihydrate has m 181-182o. Its optical rotation in MeOH decreases from [] D 25 +26o (c 0.5%) to [] D 25 +11.3o after standing for 16hours. It forms a sodium salt and a CaCl2 complex. [Regna et al. J Am Chem Soc 73 4211 1951, Beilstein 14 IV 2633.]Окситетрациклин запасные части и сырье
Окситетрациклин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18239973690 +86-18239973690 |
China | 311 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-66697723 +86-17703311139 |
China | 563 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-53169958659 +86-13153181156 |
China | 294 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +undefined17712220823 | China | 615 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8619933239880 | China | 861 | 58 |