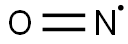
NITRIC OXIDE
- русский язык имя
- английское имяNITRIC OXIDE
- CAS №10102-43-9
- CBNumberCB5433122
- ФормулаNO*
- мольный вес30.01
- EINECS233-271-0
- номер MDLMFCD00011525
- файл Mol10102-43-9.mol
| Температура плавления | −163.6 °C(lit.) |
| Температура кипения | −151.7 °C(lit.) |
| плотность | d-150.2 (liq) 1.27; Relative d (gas) 1.036 (air = 1); Absolute d (gas) 1.227 (air = 1) |
| плотность пара | 1.05 (vs air) |
| показатель преломления | nD25 1.0002697 |
| растворимость | At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 21 volumes of water. |
| форма | colorless gas |
| цвет | colorless |
| Растворимость в воде | slightly soluble H2O [HAW93] |
| Мерк | 13,6611 |
| Пределы воздействия | TLV-TWA 25 ppm (~30 mg/m3) (ACGIH, MSHA, and OSHA). |
| Стабильность | Spontaneously reacts with oxygen in air to yield brown nitrogen dioxide. Reacts violently or explosively with ammonia and many organic materials. |
| ИнЧИКей | MWUXSHHQAYIFBG-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 10102-43-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 31C4KY9ESH |
| Код УВД | R07AX01 |
| Система регистрации веществ EPA | Nitric oxide (10102-43-9) |
| UNSPSC Code | 12352303 |
| Коды опасности | O,T | |||||||||
| Заявления о рисках | 8-23-34-44 | |||||||||
| Заявления о безопасности | 17-23-36/37/39-45 | |||||||||
| РИДАДР | UN 1660 2.3 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 25 ppm (30 mg/m3) | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | QX0525000 | |||||||||
| Классификация DOT | 2.3, Hazard Zone A (Gas poisonous by inhalation) | |||||||||
| Класс опасности | 2.3 | |||||||||
| Банк данных об опасных веществах | 10102-43-9(Hazardous Substances Data) | |||||||||
| Токсичность | LCLo inhalation in dog: 5000ppm/25M | |||||||||
| ИДЛА | 100 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H330:Смертельно при вдыхании.
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
H270:Окислитель; может вызвать или усилить возгорание.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
NITRIC OXIDE химические свойства, назначение, производство
Описание
near room temperature (its liquid density at 20°C is 1.45 g/cm3). Nitrogen monoxide (NO) is commonly called nitric oxide,Nitric oxide is colorless and has a sharp sweet odor;Nitric oxide is nonfl ammable, toxic gases.Nitric oxide is a free radical that quickly reacts in air to produce nitrogen dioxide.It is also an important biological messenger and transmitter.Химические свойства
Nitric oxide,NO, also known as nitrogen oxide and nitrogen monoxide, is a colorless gas that will react with oxygen at room temperature to form nitrogen dioxide, N202, a reddish-brown gas.It is soluble in water and alcohol and is used primarily to form other compounds.Физические свойства
Colorless gas; paramagnetic; density 1.3402 g/L; slightly heavier than air, air density 1.04 (air=1); liquefies at -151.8°C to a blue liquid; the refractive index of the liquid 1.330 at -90°C; the density of the liquid 1.269 g/mL at -150.2°C; solidifies at -163.6°C to a bluish-white snow-like solid; critical temperature -94°C; critical pressure 65 atm; slightly soluble in water, 4.6 mL gas dissolves in 100 mL water at 20°C while 7.34 mL and 2.37 mL dissolve in the same volume of water at 0 and 60°C, respectively; more soluble in alcohol than water; soluble in carbon disulfide, and in ferrous sulfate solution (reacts).История
Nitric oxide was prepared in 1772 by Joseph Priestley (1733–1804) and described in his volumes Experiments and Observations of Different Kinds of Air published between 1774 and 1786. Priestley called nitric oxide nitrous air, nitrogen dioxide nitrous acid vapor, and nitrous oxide phlogisticated nitrous air, but also referred to the latter as diminished nitrous air. He observed the change of clear nitric oxide to red nitrogen dioxide. Priestley prepared nitric oxide by reacting nitric acid with a metal such as copper: 3Cu(s) + 8HNO3(aq) → 2NO(g) + 3Cu(NO3)2(aq) + 4H2O(l).Использование
Nitric oxide is used as an intermediate in themanufacture of nitric acid, in the preparationof metal nitrosyls, in bleaching of rayon,and in incandescent lamps. It is produced byheating air at high temperatures.Методы производства
Nitric oxide is commercially produced by the catalytic oxidation of ammonia using aplatinum catalyst: 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g).Определение
ChEBI: Nitric Oxide is a nitrogen oxide which is a free radical, each molecule of which consists of one nitrogen and one oxygen atom.Общее описание
A colorless gas. Noncombustible but accelerates the burning of combustible material. Vapors heavier than air. Very toxic by inhalation and skin absorption. Heating the containers may cause them to rupture violently and rocket.Nitric oxide was discovered by Van Helmont in 1620. It occurs in the exhaust gases from automobiles along with other oxides of nitrogen, at trace concentrations. It also is found in minute quantities in the upper atmosphere, resulting from the oxidation of nitrogen in the presence of ionizing radiation or by electric discharge. Nitric oxide is the most stable oxide of nitrogen. It is used as an intermediate or as a starting reactant in the production of many nitrogen compounds, including nitrogen dioxide, nitric acid and nitrosyl chloride.
Реакции воздуха и воды
Combines very rapidly with oxygen in the air to form nitrogen dioxide. Nitrogen dioxide reacts with water to form nitric acid and NITRIC OXIDE, reacts with alkalis to form nitrates and nitrites [Merck 11th ed. 1989].Профиль реактивности
NITRIC OXIDE can serve as both an oxidizing agent and as a reducing agent. Sustains the combustion of powdered aluminum [Mellor 5:209-212. 1946-47]. Enflames or explodes when mixed with vapors of carbon disulfide [Mellor 8, Supp. 2:232. 1967]. Reacts vigorously with sodium monoxide above 100°C [Mellor 2, Supp. 2:629. 1961]. Reacts on contact with oxygen at room temperature to form brown gaseous nitrogen dioxide. Reacts with alkalis to form nitrates and nitrites [Merck 11th ed. 1989]. The liquid is very sensitive to detonation in the presence of water.Опасность
Supports combustion. Toxic by inhalation, strong irritant to skin and mucous membranes. Hypoxia/cyanosis, nitrosyl-hemoglobin formation, and upper respiratory tract irritant.Угроза здоровью
Can cause death or permanent injury after a very short exposure to small quantities. Irritant of eyes, nose, throat; can cause unconsciousness. NITRIC OXIDE forms acids in the respiratory system which are irritating and cause congestion in the lungs. Concentrations of 60-150 ppm cause immediate irritation of the nose and throat with coughing and burning in the throat and chest. 6-24 hours after exposure, labored breathing and unconsciousness may result. Concentrations of 100-150 ppm are dangerous for short exposure of 30-60 minutes. Concentrations of 200-700 ppm may be fatal after very short exposure.Пожароопасность
Noncombustible gas; burns with fuels, hydrocarbons, or when heated with hydrogen. Nitric oxide reacts violently with carbon disulfide vapors, producing green luminous flame; with fluorine, it produces a pale yel low flame. It explodes when mixed with ozone, chlorine monoxide, or a nitrogen tri halide. Reactions with many pyrophoric met als produce incandescence. Reaction with amorphous boron produces brilliant flashes.Использование в материалах
Nitric oxide is noncorrosive, and most common structural materials may be used. However, in the presence of moisture and oxygen, corrosive conditions will develop as a result of the formation of nitric and nitrous acids. Prior to use, systems to contain nitric oxide must first be purged with an inert gas. Where air contamination cannot be eliminated, stainless steel should be used.Возможный контакт
Nitric oxide is used in the manufacture of nitric acid; it is also used in the bleaching of rayon; it is a raw material for nitrosyl halide preparation.Экологическая судьба
Nitric oxide is converted spontaneously in the air to nitrogen dioxide; hence, some of the latter gas is present whenever nitric oxide is found in air (at concentrations below 50 ppm). Nitric oxide is a contributor to photochemical air pollution.хранилище
Nitric oxide should only be used in well-ventilated areas. Valve protection caps and valve outlet threaded plugs must remain in place unless the container is secured and the valve outlet piped to the point of use. Do not drag, slide, or roll cylinders. Use a suitable hand truck to move cylinders. Use a pressure reducing regulator when connecting a cylinder to lower pressure (1000 psig or 6900 kPa) piping systems. Do not heat a cylinder of nitric oxide by any means to increase the discharge rate from the cylinder. Use a check valve or trap in the discharge line to prevent hazardous reverse flow into the cylinder.Перевозки
UN1660/124 Nitric oxide, compressed, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a wellventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.Методы очистки
Bubble the gas through 10M NaOH which removes NO2. It can also be freed from NO2 by passage through a column of Ascarite followed by a column of silica gel held at -197oK. The gas is dried with solid NaOH pellets or by passing through silica gel cooled at -78o, followed by fractional distillation from a liquid N2 trap. This purification does not eliminate nitrous oxide. Other gas scrubbers sometimes used include one containing conc H2SO4 and another containing mercury. It is freed from traces of N2 by the freeze and thaw method. [Blanchard Inorg Synth II 126 1946, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 485-487 1963.] TOXIC.Несовместимости
A strong oxidizer but may also act as a reducing agent. Explosive reaction with nitrogen trichloride, ozone, carbon disulfide; pentacarbonyl iron; chlorine monoxide. Incompatible with halogens, combustibles, metals, oil, alcohols, chlorinated hydrocarbons (e.g., trichloroethylene), reducing agents (such as NH3), oxygen, fluorine, metals. Reacts with water to form nitric acid. Rapidly converted in air to nitrogen dioxide. Combines very rapidly with oxygen in the air to form nitrogen dioxide. Nitrogen dioxide reacts with water to form nitric acid and nitric oxide, reacts with alkalis to form nitrates and nitrites.Утилизация отходов
Return refillable compressed gas cylinders to supplier. Incineration with added hydrocarbon fuel, controlled so as to produce elemental nitrogen, CO2, and water. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.использованная литература
1.https://en.wikipedia.org/wiki/Nitric_oxide 2.https://www.drugs.com/mtm/nitric-oxide-inhalation-gas.html 3.https://www.britannica.com/science/nitric-oxide 4.http://pediatrics.aappublications.org/content/106/2/344 5.http://www.mensfitness.com/nutrition/supplements/supplement-guide-nitric-oxide 6.https://www.chemicalbook.com/productchemicalpropertiescb5433122.htmNITRIC OXIDE запасные части и сырье
сырьё
запасной предмет
1of3
NITRIC OXIDE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 0734-8755555 15674722888 |
China | 401 | 58 | |
| 400-6377517 19876107228 |
China | 39 | 58 | |
| +86-21-68182121 | China | 882 | 52 | |
| 021-20958197 20965099 | China | 1183 | 57 | |
| 010-56205725 | China | 12335 | 58 |