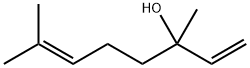
Линалоол
- английское имяLinalool
- CAS №78-70-6
- CBNumberCB5347184
- ФормулаC10H18O
- мольный вес154.25
- EINECS201-134-4
- номер MDLMFCD00008906
- файл Mol78-70-6.mol
| Температура плавления | 25°C |
| Температура кипения | 194-197 °C/720 mmHg (lit.) |
| плотность | 0.87 g/mL at 25 °C (lit.) |
| давление пара | 0.17 mm Hg ( 25 °C) |
| FEMA | 2635 | LINALOOL |
| показатель преломления | n |
| Fp | 174 °F |
| температура хранения | 2-8°C |
| растворимость | ethanol: soluble1ml/4ml, clear, colorless (60% ethanol) |
| форма | Liquid |
| пка | 14.51±0.29(Predicted) |
| Удельный вес | 0.860 (20/4℃) |
| цвет | Clear colorless to pale yellow |
| РН | 4.5 (1.45g/l, H2O, 25℃) |
| Запах | at 100.00 %. citrus floral sweet bois de rose woody green blueberry |
| Odor Type | floral |
| Биологические источники | synthetic |
| Пределы взрываемости | 0.9-5.2%(V) |
| Растворимость в воде | 1.45 g/L (25 ºC) |
| Мерк | 14,5495 |
| Номер JECFA | 356 |
| БРН | 1721488 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. Combustible. |
| ИнЧИКей | CDOSHBSSFJOMGT-UHFFFAOYSA-N |
| LogP | 2.9 at 20℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | LINALOOL |
| FDA 21 CFR | 182.60 |
| Справочник по базе данных CAS | 78-70-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2-5 |
| FDA UNII | D81QY6I88E |
| Справочник по химии NIST | 2,6-Dimethylocta-2,7-dien-6-ol(78-70-6) |
| Пестициды Закон о свободе информации (FOIA) | Linalool |
| Система регистрации веществ EPA | 3,7-Dimethyl-1,6-octadien-3-ol (78-70-6) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | Xi,Xn | |||||||||
| Заявления о рисках | 36/37/38-20/21/22 | |||||||||
| Заявления о безопасности | 26-36 | |||||||||
| РИДАДР | NA 1993 / PGIII | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | RG5775000 | |||||||||
| Температура самовоспламенения | 235 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29052210 | |||||||||
| Банк данных об опасных веществах | 78-70-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2790 mg/kg LD50 dermal Rabbit 5610 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Линалоол химические свойства, назначение, производство
Описание
Linalool has a typical floral odor free from camphoraceous and terpenic notes.1 Synthetic linalool exhibits a cleaner and fresher note than the natural product. It can be prepared synthetically starting from myrcene or from dehydrolinalool.The optically active forms (d- and ι-) and the optically inactive form occur naturally in more than 2 0 0 oils from herbs, leaves, flowers, and wood; the ι-form is present in the largest amounts (80 - 85%) in the distillates from leaves of Cinnamomum cam phora var. orientalis and Cinnamomum camphora var. occidentalis and in the distillate from Cajenne rosewood; it also has been reported in: champaca, ylang-ylang, neroli, Mexican linaloe, ber gamot, lavandin, and others; a mixture of d- and ι-linalool has been reported in Brazil rosewood (85%); the d-form has been found in palmarosa, mace, sweet orange-flower distillate, petit grain, coriander (60 - 70%), marjoram, Orthodon linalooliferum (80%), and others; the inactive form has been reported in clary sage, jasmine, and Nectandra elaiophora.
Химические свойства
Linalool occurs as one of its enantiomers in many essential oils, where it is often the main component. (3R)- (?)-Linalool, for example, occurs at a concentration of 80–85% in Ho oils from Cinnamomum camphora; rosewood oil contains about 80%. (3S)-(+)- Linalool makes up 60–70% of coriander oil (“coriandrol”).Linalool is used frequently in perfumery for fruity notes and for many floral fragrance compositions (lily of the valley, lavender, and neroli). Because of its relatively high volatility, it imparts naturalness to top notes. Since linalool is stable in alkali, it can be used in soaps and detergents. Linalyl esters can be prepared from linalool.Most of the manufactured linalool is used in the production of vitamin E.
Физические свойства
Properties. Racemic linalool is, similarly to the individual enantiomers, a colorless liquid with a floral, fresh odor, reminiscent of lily of the valley. However, the enantiomers differ slightly in odor. Together with its esters, linalool is one of the most frequently used fragrance substances and is produced in large quantities. In the presence of acids, linalool isomerizes readily to geraniol, nerol, and α-terpineol. It is oxidized to citral, for example, by chromic acid. Oxidation with peracetic acid yields linalool oxides, which occur in small amounts in essential oils and are also used in perfumery. Hydrogenation of linalool gives tetrahydrolinalool, a stable fragrance material. Its odor is not as strong as, but is fresher than, that of linalool. Linalool can be converted into linalyl acetate by reaction with ketene or with an excess of boiling acetic anhydride.Вхождение
The optically active forms (d- and l-) and the optically inactive form occur naturally in more than 200 oils from herbs, leaves, flowers and wood; the l-form is present in the largest amounts (80 to 85%) in the distillates from leaves of Cinnamomum camphora var. orientalis and Cinnamomum camphora var. occidentalis and in the distillate from Cajenne rosewood; it also has been reported in champaca, ylang-ylang, neroli, Mexican linaloe, bergamot and lavandin; a mixture of d- and l-linalool has been reported in Brazil rosewood (85%); the d-form has been found in palmarosa, mace, sweet orange-flower distillate, petitgrain, coriander (60 to 70%), marjoram and Orthodon linalooliferum (80%); the inactive form has been reported in clary sage, jasmine and Nectandra elaiophora. Also reported found in over 280 products including apple, citrus peel oils and juices, berries, grapes, guava, celery, peas, potato, tomato, cinnamon, cloves, cassia, cumin, ginger, mentha oils, mustard, nutmeg, pepper, thymus, cheeses, grape wines, butter, milk, rum, cider, tea, passion fruit, olive, mango, beans, coriander, cardamom and rice.Использование
linalool is a fragrant component of both lavender and coriander. It can be incorporated into cosmetics for perfuming, deodorant, or odor-masking activity.Определение
ChEBI: A monoterpenoid that is octa-1,6-diene substituted by methyl groups at positions 3 and 7 and a hydroxy group at position 3. It has been isolated from plants like Ocimum canum.Общее описание
Linalool is a monoterepene compound. It is the major component of many essential oils. Anti-inflammatory properties of (?) linalool, a naturally occurring enantiomer, has been studied. It is also the major constituent of the basil and thyme essential oil and its anti-microbial effect was determined using the agar well diffusion assay. Linalool is reported to have dose-dependent marked sedative effects at the central nervous system (CNS), including hypnotic, anticonvulsant and hypothermic properties. Linalool is reported to have a lemon like odor.Контактные аллергены
Linalool is a terpene chief constituent of linaloe oil,also found in oils of Ceylon cinnamon, sassafras,orange flower, bergamot, Artemisia balchanorum, ylang-ylang. This frequently used scented substance is a sensitizer by the way of primary or secondary oxida-tion products. As a fragrance allergen, linalool has to be mentioned by name in cosmetics within the EUразработк И противоопухолевых препаратов
Studies of antitumor activities and toxicity were done on solid S-180 tumor-bearingSwiss albino mice. It results in an induction of oxidative stress with an antitumoractivities result. In comparison with cyclophosphamide, antioxidant effects wereobserved in the liver and modulation of proliferation of spleen cells in tumor-bearingmice challenged with lipopolysaccharides, while both were seriously affected bycyclophosphamide (Costa et al. 2015).Линалоол запасные части и сырье
сырьё
1of4
запасной предмет
1of3
Линалоол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | |
| +8615318402391 | China | 809 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +86-13734021967 +8613734021967 |
China | 1001 | 58 |