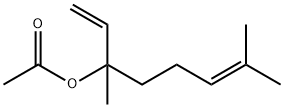
Линaлил ацета
- английское имяLinalyl acetate
- CAS №115-95-7
- CBNumberCB0674881
- ФормулаC12H20O2
- мольный вес196.29
- EINECS204-116-4
- номер MDLMFCD00008907
- файл Mol115-95-7.mol
| Температура плавления | 85°C |
| Температура кипения | 220 °C(lit.) |
| плотность | 0.901 g/mL at 25 °C(lit.) |
| плотность пара | 6.8 (vs air) |
| давление пара | 0.1 mm Hg ( 20 °C) |
| FEMA | 2636 | LINALYL ACETATE |
| показатель преломления | n |
| Fp | 194 °F |
| температура хранения | -20°C |
| растворимость | Chloroform (Slightly), Methanol (Slightly) |
| форма | Liquid |
| цвет | Clear colorless |
| Запах | at 100.00 %. sweet green citrus bergamot lavender woody |
| Odor Type | herbal |
| оптическая активность | (S)-:-9.4520 |
| Биологические источники | synthetic |
| Растворимость в воде | 499.8mg/L(25 ºC) |
| Мерк | 14,5496 |
| Номер JECFA | 359 |
| БРН | 1724500 |
| ИнЧИКей | UWKAYLJWKGQEPM-LBPRGKRZSA-N |
| LogP | 3.9 at 25℃ |
| Справочник по базе данных CAS | 115-95-7(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | LINALYL ACETATE |
| FDA 21 CFR | 182.60 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 5K47SSQ51G |
| Справочник по химии NIST | 1,6-Octadien-3-ol, 3,7-dimethyl-, acetate(115-95-7) |
| Система регистрации веществ EPA | Linalyl acetate (115-95-7) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38-38 | |||||||||
| Заявления о безопасности | 26-36-37-24/25 | |||||||||
| РИДАДР | NA 1993 / PGIII | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | RG5910000 | |||||||||
| кода HS | 29153900 | |||||||||
| Банк данных об опасных веществах | 115-95-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 13934 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Линaлил ацета химические свойства, назначение, производство
Химические свойства
Linalyl Acetate occurs as its isomer as the main component of lavender oil (30–60%, depending on the origin of the oil), of lavandin oil (25–50%, depending on the species), and of bergamot oil (30–45%). It has also been found in clary sage oil (up to 75%) and in a small amount in many other essential oils. Racemic linalyl acetate is a colorless liquid with a distinct bergamot–lavender odor.
Linalyl acetate is used extensively in perfumery. It is an excellent fragrance material for, among others, bergamot, lilac, lavender, linden, neroli, ylang-ylang, and fantasy notes (particularly chypre). Smaller amounts are used in other citrus products. Since linalyl acetate is fairly stable toward alkali, it can also be employed in soaps and detergents.
Вхождение
Reported found in the essential oils of bergamot, lavender, clary sage and lavandin; also identified among the constituents of the essential oils of Salvia officinalis, petitgrain, sassafras, neroli, lemon, Italian lime, jasmine, Mentha citrata, Mentha aquatica, Thymus mastichina, etc; also reported in abundant quantities in the essential oil from flowers, leaves and stems of Tagetes patula, in the distillate from leaves of Citrus aurantifolia from India, and in the essential oil of Mentha arvensis. Also reported found in citrus peel oils and juices, berries, peach, celery, tomato, cinnamon, clove, nutmeg, pepper, thymus, grape wines, avocado, mushroom, marjoram, mango, cardamom, coriander, gin, origanum, lovage, laurel, myrtle leaf, rosemary, sage and mastic gum oil.Использование
Linalyl Acetate ermentative production of medium or short chain length alcohol, esters and/or glucosides by metabolically engineered microorganism.Подготовка
Linalyl acetate is synthesized by two methods:1) Esterification of linalool requires special reaction conditions since it tends to undergo dehydration and cyclization as it is an unsaturated tertiary alcohol. These reactions can be avoided as follows: esterification with ketene in the presence of an acidic esterification catalyst below 30 °C results in the formation of linalyl acetate without any by-products. Esterification can be achieved in good yield, with boiling acetic anhydride, whereby the acetic acid is distilled off as it is formed; a large excess of acetic anhydride must be maintained by continuous addition of anhydride to the still vessel. Highly pure linalyl acetate can be obtained by transesterification of tert-butyl acetate with linalool in the presence of sodium methylate and by continuous removal of the tert-butanol formed in the process.
2) Dehydrolinalool, obtained by ethynylation of 6-methyl-5-hepten-2-one, can be converted into dehydrolinalyl acetatewith acetic anhydride in the presence of an acidic esterification catalyst. Partial hydrogenation of the triple bond to linalyl acetate can be carried out with, for example, palladium catalysts deactivated with lead.
Общее описание
Linalyl acetate is a monoterpene ester mainly found in lavandula essential oil. It is used as a flavoring agent in food industries, as well as a preservative additive in cosmetics and fragrances such as soaps, colognes, perfumes, and skin lotions.Контактные аллергены
Structurally close to linalool, linalyl acetate is the main component of lavender oil and is commonly used in fragrances and toiletries, and in household cleaners and detergents as well. By autoxidation, it leads mainly to hydroperoxides, with a high sensitizing potent.Линaлил ацета запасные части и сырье
Линaлил ацета поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8613223293093 | China | 80 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +8613288715578 | China | 1174 | 58 | |
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18017610038 | CHINA | 3619 | 58 |