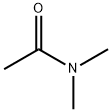
N,N-диметилацетамид
- английское имяN,N-Dimethylacetamide
- CAS №127-19-5
- CBNumberCB8853004
- ФормулаC4H9NO
- мольный вес87.12
- EINECS204-826-4
- номер MDLMFCD00008686
- файл Mol127-19-5.mol
| Температура плавления | -20 °C (lit.) |
| Температура кипения | 164.5-166 °C (lit.) |
| плотность | 0.937 g/mL at 25 °C (lit.) |
| плотность пара | 3.89 (vs air) |
| давление пара | 40 mm Hg ( 19.4 °C) |
| показатель преломления | n |
| Fp | 158 °F |
| температура хранения | Store below +30°C. |
| растворимость | >1000g/l soluble |
| форма | Liquid |
| пка | -0.41±0.70(Predicted) |
| цвет | Colorless to yellowish |
| РН | 4 (200g/l, H2O, 20℃) |
| Относительная полярность | 6.3 |
| Запах | Faint ammonia odor |
| Водородный показатель | 4 at 200 g/l at 20 °C |
| Скорость испарения | <0.17 |
| Порог?обнаружения?запаха? | 0.76ppm |
| Пределы взрываемости | 1.7-11.5%(V) |
| Растворимость в воде | miscible |
| λмакс | λ: 270 nm Amax: 1.00 λ: 280 nm Amax: 0.30 λ: 290 nm Amax: 0.15 λ: 310 nm Amax: 0.05 λ: 320 nm Amax: 0.03 λ: 360-400 nm Amax: 0.01 |
| Мерк | 14,3227 |
| БРН | 1737614 |
| Пределы воздействия | NIOSH REL: TWA 10 ppm (35 mg/m3), IDLH 300 ppm; OSHA PEL: TWA 10 ppm; ACGIH TLV: TWA 10 ppm (adopted). |
| Диэлектрическая постоянная | 37.8(25℃) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | FXHOOIRPVKKKFG-UHFFFAOYSA-N |
| LogP | -0.77 at 25℃ |
| Справочник по базе данных CAS | 127-19-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | JCV5VDB3HY |
| Предложение 65 Список | N,N-Dimethylacetamide |
| Справочник по химии NIST | Acetamide, N,N-dimethyl-(127-19-5) |
| МАИР | 2B (Vol. 123) 2020 |
| Система регистрации веществ EPA | Dimethylacetamide (127-19-5) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T | |||||||||
| Заявления о рисках | 11-20/22-61-20/21-36 | |||||||||
| Заявления о безопасности | 16-24-53-45-36-28-26 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 10 ppm (35 mg/m3) [skin] | |||||||||
| RTECS | AB7700000 | |||||||||
| F | 3-10 | |||||||||
| Температура самовоспламенения | 914 °F | |||||||||
| кода HS | 29241900 | |||||||||
| Банк данных об опасных веществах | 127-19-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 5.4 ml/kg (Bartsch) | |||||||||
| ИДЛА | 300 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H360D:Может отрицательно повлиять на неродившегося ребенка.
H312+H332:Вредно при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
N,N-диметилацетамид химические свойства, назначение, производство
Описание
Dimethylacetamide (DMAC) is a synthetic organic compound that is produced from a reaction of dimethylamine and acetic acid or acetic anhydride. It is a colorless to yellow liquid with a faint odor resembling ammonia. DMAC has similar density to water and is miscible with water and organic substances. This organic compound is commonly associated with many industrial uses, either as a starting material or an intermediate. DMAC is a good solvent that is used in polymer dissolution, especially in the fiber industry. Historically, DMAC was also tested as a possible antineoplastic agent in a phase 1 study involving 17 patients. However, liver and central nervous system (CNS) toxicity associated with DMAC was observed and these patients had altered mental states, resulting in no further drug development.Химические свойства
Dimethylacetamide occurs as a clear, colorless, slightly hygroscopic liquid with a weak ammonia-like or fish-like odor. odor threshold concentration is 46.8 ppmv (Leonardos et al., 1969). It is soluble in a wide variety of solvents.Использование
N,N-dimethylacetamide is an excellent solvent and often acts as a catalyst in halogenation, cyclization, and alkylation reactions. It is an important industrial solvent for polyacrylonitrile, vinyl resins, cellulose derivatives, styrene polymers and linear polyesters. It is also used in all analytical laboratories as a major component of mobile phase in High Performance Liquid Chromatography (HPLC) and in Liquid Chromatography - Mass Spectrometry (LC-MS). N,N-dimethylacetamide lithium chelate complexes intercalate cationic sites in layered silicates.прикладной
N,N-Dimethylacetamide is primarily used as an industrial solvent and intermediate in the manufacture of pharmaceuticals, fine chemicals, agrochemicals, polymers, and resins. It is also used as a spinning solvent in the production of fibers of various polymers, including acrylic, polyurethane, polyurea copolymer, and meta-aramid. Moreover, this aprotic dipolar solvent is also used in X-ray and photographic products and in the production of polyimide films. The polyimide films are produced for a variety of industries, including consumer electronics, solar photovoltaic and wind energy, aerospace, automotive, and industrial applications. N,N-Dimethylacetamide has other minor uses, including removal of ink, stripping of paint, and also for laboratory use.Определение
ChEBI: N,N-dimethylacetamide is a member of the class of acetamides that is acetamide in which the hydrogens attached to the N atom have been replaced by two methyl groups respectively. Metabolite observed in cancer metabolism. It has a role as a human metabolite. It is a member of acetamides and a monocarboxylic acid amide. It is functionally related to an acetamide.Подготовка
N,N-Dimethylacetamide is prepared by reaction of dimethylamine with acetic acid, acetic anhydride, or acetate esters. Heating dimethylamine acetate with, or without a catalyst affords N,N-Dimethylacetamide. Reaction of dimethylamine with acetate esters requires a catalyst; sodium methoxide is typically used.Общее описание
Dimethylacetamide appears as a clear colorless liquid with a faint odor similar to ammonia. About the same density as water. Flash point 145°F. Vapors heavier than air. May by toxic by skin absorption. May irritate eyes and skin.Реакции воздуха и воды
Water soluble.Профиль реактивности
N,N-Dimethylacetamide is an amide. Incompatible with oxidizing agents and halogenated compounds. Exothermic reactions occur with carbon tetrachloride and hexachlorocyclohexane. N,N-Dimethylacetamide can react violently in the presence of iron. Special Hazards of Combustion Products: Emits carbon oxides, nitrogen oxides, and dimethylamine when heated to decomposition.Угроза здоровью
A study of 41 workers who had been exposed to dimethylacetamide from 2 to 10 years revealed the occurrence of disorders reflecting liver damage (Corsi, 1971). Retention of bromosulfophthalein was increased in 9 of 10 workers who had been exposed to dimethylacetamide for 7 to 10 years, and in 10 of 20 workers who had been exposed to dimethylacetamide for 2 to 7 years. Other parameters of hepatic function which were altered in the exposed individuals include proteinemia, cholesterolemia, activities of hepatic transaminases and alkaline phosphatase in serum, and bilirubinemia. Hepatomegaly was diagnosed in 14 workers.In a clinical trial, dimethylacetamide was administered to patients with advanced malignancies and caused abnormal mental states (Weiss et al 1962a, b). This effect was observed at a dose of 400 mg/kg given daily for 3 or more days.
The symptoms were not seen at 300 mg/kg or lower. The second or third dose caused depression, lethargy, occasional confusion or disorientation. The fourth or fifth dose produced striking hallucinations, perceptual distortions and delusions in all nine patients. All patients reverted to normal several days after discontinuation of treatment with dimethylacetamide.
Пожароопасность
N,N-Dimethylacetamide is combustible.Промышленное использование
Dimethylacetamide is a powerful industrial solvent, the uses of which are very similar to those of dimethylformamide (Siegle, 1980). Its strong solvent action renders it particularly useful in the manufacture of films and fibers and as a solvent for polyacrylonitrile, polyvinyl chloride, polyamides, cellulose derivatives and polystyrenes and in coatings and adhesive formulations. Dimethylacetamide dissolves many inorganic salts.Профиль безопасности
Moderately toxic by skin contact, inhalation, intravenous, and intraperitoneal routes. Mildly toxic by ingestion. Experimental teratogenic and reproductive effects. A skin and eye irritant. Less toxic than dimethylformamide. Mutation data reported. Combustible when exposed to heat and flame. A moderate explosion hazard. Violent reaction with halogenated compounds (e.g., carbon tetrachloride, hexachlorocyclohexane) when heated above 9OOC. Iron powder catalyzes the reaction so that it initiates at 71OC. When heated to decomposition it emits toxic fumes of NOxБезопасность
Dimethylacetamide is used in pharmaceutical preparations as a solvent in parenteral formulations and is generally regarded as a nontoxic material when used as an excipient. Animal toxicity studies indicate that dimethylacetamide is readily absorbed into the bloodstream following inhalation or topical application. Repeated exposure to dimethylacetamide may be harmful and can result in liver damage. High intravenous doses (>400mg/kg/day for 3 days) may be hallucinogenic.LD50 (rabbit, SC): 9.6g/kg(11) LD50 (rat, IP): 2.75g/kg LD50 (rat, IV): 2.64g/kg LD50 (rat, oral): 4.93g/kg LD50 (mouse, inhalation): 7.2g/kg LD50 (mouse, IP): 2.8g/kg
Описание
N,N-диметилацетамид— бесцветная или бледно-желтая жидкость. Растворим в воде, органических растворителях. Предельно допустимая концентрация 10 мг/м3.Канцерогенность
DMAC was not carcinogenic in rats administered 100, 300, or 1000 mg/kg/day in drinking water for 2 years. Rats and mice were exposed by inhalation to 0, 25, 100, or 350 ppm DMAC for 6 h/day, 5 days/week for 18 months (mice) or 2 years (rats). DMAC was not oncogenic under these conditions in either the rat or the mouse.Применение
N,N-Диметилацетамид применятеся при производстве синтетических волокон и пленок, для выделения диенов и стирола из продуктов пирролиза нефтянных фракций. В органическом синтезе диметилацетамид широко используется в качестве растворителя, а также в качестве реагента при ацилировании некоторых соединений.Экологическая судьба
Chemical/Physical. Releases toxic fumes of nitrogen oxides when heated to decomposition (Sax and Lewis, 1987).Метаболизм
33 mg or 92 mg of 14C-labelled DMAC were given by gavage to two rats. The major urinary components were:60-70% MMAC, 7-10% N -hydroxymethylacetamide, 7- 10%, Acetamide and some residual DMAC. A small proportion of DMAC and its intermediates was hydrolysed and eliminated as Carbon Dioxide.Twenty male rats were given two subcutaneous injections of 300 mg DMAC, MMAC and Acetamide were found in the urine.
20 rats had 20-80% DMAC solutions (0.12 ml) applied to their backs. The amount of MMAC increased as the solution concentration increased. Recovery ranged from 13% for the 20% solution to 42% for undiluted DMAC (Du Pont, 1988).
These studies show that the major metabolic pathway of DMAC "in vivo" in rats is sequential N-demethylation and elimination from the body.
https://hpvchemicals.oecd.org/ui/handler.axd?id=16564422-daff-4489-8c27-735d3ab0b260
Производство
Диметилацетамид получают взаимодействием уксусной кислоты или ее ангидрида с диметиламином.хранилище
Dimethylacetamide should be stored in an airtight container, protected from light, in a cool, dry place. Dimethylacetamide has an almost unlimited shelf-life when kept in closed containers and under nitrogen. It is combustible.Методы очистки
Shake the amide with BaO for several days, reflux it with BaO for 1hour, then fractionally distil it under reduced pressure. Store it over molecular sieves. [Beilstein 4 IV 180.]Несовместимости
Dimethylacetamide is incompatible with carbon tetrachloride, oxidizing agents, halogenated compounds, and iron. It attacks plastic and rubber. Contact with strong oxidizers may cause fire.Регуляторный статус
Included in the FDA Inactive Ingredients Database (IM injections, IV injections and infusions). Included in parenteral medicines licensed in the UK.N,N-диметилацетамид запасные части и сырье
сырьё
1of2
запасной предмет
- 1,2-дихлорпропана
- Molosultap
- 1-(5-НИТРО-3-ТИЕНИЛ)ЭТАН-1-ОН
- 1H-ПИРРОЛО[2,3-B]ПИРИДИН, 2-МЕТИЛ-
- Цефотаксим натрия
- 2-(1-ГИДРОКСИЛАМИНОЭТИЛ)-БЕНЗОТИОФЕН
- 2-Acetylbenzo [B] тиофен
- 3-(Benzofuran-2-yl)-3-oxopropanenitrile ,97%
- Флуоксетин
- 1-Нафталинсульфонилхлорид
- 5-ацетилиндол
- complex polystyrene high efficiency anticorrosion paint
- Перголид
- 1,3-дихлорпропен
- Thiocyclam hydrogen oxalate
- Цефоперазон
- 6-БРОМЕТИЛ-ПТЕРИДИН-2,4-ДИАМИН HBR
- 2-Ацетил-3-аминотиофена
- 4-Hydroxyindole
- 4'-Phenoxyacetophenone
1of7
N,N-диметилацетамид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +8613573296305 | China | 301 | 58 | ||
| +8617653113209 | China | 3049 | 58 | ||
| +8613325218432 | China | 57 | 58 | ||
| +86-85511178; +86-85511178; |
China | 35425 | 58 | ||
| +86-533-2119995 +8615053330977 |
China | 37 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 |
N,N-диметилацетамид Обзор)
1of4