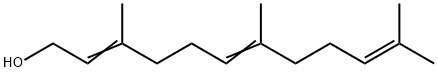
Фарнесол
- английское имяFARNESOL
- CAS №4602-84-0
- CBNumberCB7669246
- ФормулаC15H26O
- мольный вес222.37
- EINECS225-004-1
- номер MDLMFCD00002918
- файл Mol4602-84-0.mol
| Температура плавления | 25°C |
| Температура кипения | 149 °C4 mm Hg(lit.) |
| плотность | 0.886 g/mL at 20 °C(lit.) |
| давление пара | 13Pa at 20℃ |
| FEMA | 2478 | FARNESOL |
| показатель преломления | n |
| Fp | 205 °F |
| температура хранения | 2-8°C |
| растворимость | DMSO:100.0(Max Conc. mg/mL);449.7(Max Conc. mM) |
| пка | 14.69±0.10(Predicted) |
| форма | Liquid |
| цвет | Clear colorless to faint yellow |
| Запах | at 100.00 %. mild fresh sweet linden floral angelica |
| Odor Type | floral |
| Биологические источники | synthetic |
| Растворимость в воде | Not miscible or difficult to mix in water. |
| Чувствительный | Light Sensitive |
| Мерк | 14,3937 |
| Номер JECFA | 1230 |
| БРН | 1763926 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| LogP | 4.72 at 22.3℃ |
| Справочник по базе данных CAS | 4602-84-0(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | FARNESOL |
| Рейтинг продуктов питания EWG | 2-4 |
| FDA UNII | EB41QIU6JL |
| Справочник по химии NIST | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-(4602-84-0) |
| Система регистрации веществ EPA | Farnesol (4602-84-0) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | Xi | |||||||||
| Заявления о безопасности | 24/25-22 | |||||||||
| РИДАДР | UN 3082 9 / PGIII | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | JR4979000 | |||||||||
| F | 8 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29052200 | |||||||||
| Банк данных об опасных веществах | 4602-84-0(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P272:Не уносить загрязненную спецодежду с места работы.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
Фарнесол химические свойства, назначение, производство
Описание
Farnesol has a characteristic flowery odor.Химические свойства
Farnesol is a component of many blossom oils. It is a colorless liquid with a linden blossom odor, which becomes more intense when evaporated.Of the four possible isomers (due to the double bonds in the 2- and 6-positions), the (2E,6E)-isomer is the most common in nature and occurs, forexample, in ambrette seed oil.(2Z,6E)-Farnesol [3790-71-4] has been identified in petitgrain oil bigarade.
Synthetic farnesol is a mixture of isomers obtained by isomerization of nerolidol. Farnesol is particularly suited for use in flower compositions and is valued for its fixative and deodorizing properties.
Вхождение
The presence of this terpene alcohol in nature has been reported in more than 30 essential oils; the levels are generally low (0.5 to 1.0%) with the exception of cabreuva, which contains up to 2 5% farensol, and the distillate from fowers of Oxystigma buccholtzii Harms which contains up to 18% farnesol Among the essential oils containing farnesol are lemongrass, Ceylon citronella, cananga, ambrette seeds, ylang-ylang, Acacia farnesiana, Peru balsam, palmarosa, tuberose, and others Reported found in apricot, citrus peel oils, grapefruit juice, strawberry jam, ginger, clove bud, hop oil, cardamom, ginger, thyme, beer, whiskey, basil, papaya, anise seed, German chamomile and Cympogon citratus oilsИспользование
farnesol is described as a substance of high biological potential, capable of acting in the skin as a true bioactivator. A biological precursor and fatty alcohol, farnesol is one component of vitamin K. It is said to help smooth wrinkles, normalize sebum secretion, and increase the skin’s elasticity, tissue tension, and moisture-binding capability. It is able to penetrate the epidermis. In humans, farnesol is found in the skin and is involved in sterol biosynthesis. It is also used for its deodorant, odor-masking, and skin-soothing properties. In clinical studies, farnesol has demonstrated anti-microbial activity, though it is unclear if this remains the case once incorporated into a cosmetic formulation. It is widely present in vegetables and found in many essential oils (for example, acacia, lilac, lily of the valley, rose, orange blossom, oak moss, and sandalwood).Определение
ChEBI: A farnesane sesquiterpenoid that is dodeca-2,6,10-triene substituted by methyl groups at positions 3, 7 and 11 and a hydroxy group at position 1.Подготовка
One method uses cabreuva as the starting material (Swiss Patent 261,120-Givaudan and Co ), while a second method starts from ambrette seeds (German Patent 149, 603-Haarmann and Reimer).Общее описание
Colorless liquid with a delicate floral odor.Профиль реактивности
Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.Контактные аллергены
Farnesol is one of the most frequent contact allergens in perfumes. It is contained in small amounts in Myroxylon pereirae and poplar buds. It is a blend of four diastereosiomers trans/cis. As a fragrance allergen, farnesol has to be mentioned by name in cosmetics within the EU.разработк И противоопухолевых препаратов
A pharmacogenomic approach was used for the farnesol. Tests on many genesinvolved in apoptosis, regulation of transcription, and genes like INE1, CTRL,MRS2, NEB, LMO7, C9orf3, and EHBP1 are not conferred resistance to farnesol.The effects of farnesol on genes not related to the resistance to anticancer drugs mayspeculate the design of new drugs against tumor-resistant line (Manjamalai andGrace 2012a, 2012b; Ji et al. 2014).Методы очистки
The main impurity is the cis-trans isomer. Purify it by gas chromatography using a 4ft x 0.125in 3%OV-1 column at 150o. [Corey et al. J Am Chem Soc 92 6637 1970, Popjak et al. J Biol Chem 237 56 1962.] It has also been fractionated through a 14-in Podbielniak column (p 11) at 11o/0.35mm. Alternatively it has been purified by gas chromatography using SF96 silicone on Fluoropak columns or Carbowax 20M on Fluoropak or base-washed 30:60 firebrick (to avoid decomposition, prepared by treating the firebrick with 5N NaOH in MeOH and washed with MeOH to pH 8) at 210o with Helium carrier gas at 60 mL/min flow rate. The diphenylcarbamoyl derivative has m 61-63o (from MeOH) and has an IR band at 3500 cm-1. [Bates et al. J Org Chem 28 1086 1963, Beilstein 1 IV 2335.]Фарнесол запасные части и сырье
сырьё
запасной предмет
Фарнесол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 |