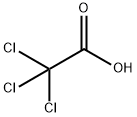
Трихлоруксусная кислота
- английское имяTrichloroacetic acid
- CAS №76-03-9
- CBNumberCB9854255
- ФормулаC2HCl3O2
- мольный вес163.39
- EINECS200-927-2
- номер MDLMFCD00004177
- файл Mol76-03-9.mol
| Температура плавления | 54-58 °C (lit.) |
| Температура кипения | 196 °C (lit.) |
| Плотность накопления | 900kg/m3 |
| плотность | 1.62 g/mL at 25 °C (lit.) |
| плотность пара | <1 (vs air) |
| давление пара | 1 mm Hg ( 51 °C) |
| показатель преломления | n |
| Fp | 196°C |
| температура хранения | Store at +15°C to +25°C. |
| растворимость | H2O: 0.5 M at 20 °C, clear, colorless |
| форма | Solid |
| пка | 0.7(at 25℃) |
| цвет | White |
| РН | <1.0 (25℃, 0.5M in H2O) |
| Запах | sharp, pungent odor |
| Растворимость в воде | 120 g/100 mL (20 ºC) |
| Чувствительный | Hygroscopic |
| λмакс | 210nm(EtOH)(lit.) |
| Мерк | 14,9627 |
| БРН | 970119 |
| Диэлектрическая постоянная | 4.9(20℃) |
| Пределы воздействия | ACGIH: TWA 0.5 ppm NIOSH: TWA 1 ppm(7 mg/m3) |
| Стабильность | Stable, but moisture sensitive. Incompatible with water, strong bases. Note that the Merck Index states that this material is hydrolytically unstable in aqueous solution below 30% by weight. Decomposition products include carbon monoxide and carbon dioxide. The generation of these gases in a sealed container may lead to a pressure rise sufficien |
| LogP | 1.330 |
| Справочник по базе данных CAS | 76-03-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3-6 |
| FDA UNII | 5V2JDO056X |
| Предложение 65 Список | Trichloroacetic Acid |
| МАИР | 2B (Vol. 63, 84, 106) 2014 |
| Справочник по химии NIST | Acetic acid, trichloro-(76-03-9) |
| Система регистрации веществ EPA | Trichloroacetic acid (76-03-9) |
| UNSPSC Code | 41105319 |
| NACRES | NB.22 |
| Коды опасности | Xn,N,C,F,Xi | |||||||||
| Заявления о рисках | 36/37/38-40-51/53-50/53-35-38-11-34-67-52/53 | |||||||||
| Заявления о безопасности | 26-36/37-61-60-45-36/37/39-24/25-16 | |||||||||
| РИДАДР | UN 1839 8/PG 2 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 1 ppm (7 mg/m3) | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | AJ7875000 | |||||||||
| F | 10-21 | |||||||||
| Температура самовоспламенения | 711 °C | |||||||||
| Примечание об опасности | Corrosive/Hygroscopic | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29154000 | |||||||||
| Банк данных об опасных веществах | 76-03-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 5000 mg/kg (Bailey, White) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H335:Может вызывать раздражение верхних дыхательных путей.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Трихлоруксусная кислота химические свойства, назначение, производство
Описание
Trichloroacetic acid, also known as TCA or 76-03-9, is a colorless or white orthorhombic crystal with strong deliquescence and a slight, special irritant smell. It is highly corrosive. TCA's aqueous solution is strongly acidic, with a pH of 1.2 for a 0.1 mol solution. Concentrations of TCA at or below 30% cannot be stored for long periods due to decomposition into chloroform, hydrogen chloride, carbon dioxide, and carbon monoxide. Dilute alkali leads to hydrolysis into chloroform and carbon dioxide. Concentrated alkali results in the formation of formic acid. TCA is a highly toxic substance with an oral LD50 of 3320mg/kg.Химические свойства
Trichloroacetic acid, a colorless crystalline solid, is commonly utilized in liquid solutions. It has the ability to absorb moisture from the surrounding air and become syrupy. When dissolved in water, the process releases heat. However, this potent acid is corrosive to both metals and tissue.Использование
Protein precipitation reagentTrichloroacetic acid is used as a precipitating agent in biochemistry for precipitation of proteins, DNA and RNA. It is an active ingredient used in cosmetic treatments like chemical peels, tattoo removal and the treatment of warts including genital warts. It is also used to determine protein concentration and as a decalcifier and fixative in microscopy.прикладной
Trichloroacetic acid(76-03-9) can be used as pharmaceutical raw materials, herbicides (potassium trichloroacetate and sodium trichloroacetate, etc.), textile dyeing auxiliaries, metal surface treatment agent and acid chloride, anhydride, amide, polyester, organometallic salt, water salicylaldehyde, chlorocarboxylic acid and the raw materials of other organic synthesis.In addition, in medicine, it can also be used as etherifying agents and keratolytics, bile pigment reagents and protein precipitation reagents. In the field of biochemistry, it can be used for separation analysis of biological phosphate compounds and reagents for determination of fluoride and lipid as well as microscopic fixative, decalcification, chromatography reagents.
The product is warts agent and astringent in pharmaceutical field, mainly used as biochemical drug extractant for the extraction of many highly efficient drugs such as adenosine triphosphate, cytochrome C and placental polysaccharides.
In addition, trichloroacetic acid, together with alkaline phenol, can be used for salicylaldehyde compound synthesis by ReimerTiemann reaction. It can also react with monoolefine compounds for synthesizing chlorocarboxylic acid [CCl3 (CH2CH2) nCOOH].
Определение
ChEBI: Trichloroacetic acid is a monocarboxylic acid that is acetic acid in which all three methyl hydrogens are substituted by chlorine. It has a role as a metabolite, a carcinogenic agent and a mouse metabolite. It is a monocarboxylic acid and an organochlorine compound. It is functionally related to an acetic acid. It is a conjugate acid of a trichloroacetate.Реакции
Trichloroacetic acid is a strong organic acid with a dissociation constant K = 3 × 10-2. It has lively chemical properties. Its sodium salt is easily subject to decarboxylation into chloroform. It will be reduced to alcohol upon coming across LiAlH4. It can have halogen replacement reaction with KBr: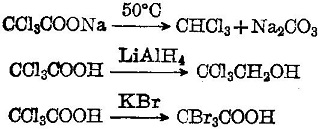
Общее описание
Trichloroacetic acid (TCA) is derived from Trichloroethylene (TCE) metabolism. It is used as an acid decalcifying agent. TCA is used as a fixative for nuclear staining and protein precipitation.Профиль реактивности
Trichloroacetic acid is a strong acid; when heated, in the presence of water, decomposes forming phosgene and HCl. [Handling Chemicals Safely 1980 p. 915]. The acid was added to copper wool and rinsed down with dimethyl sulfoxide. This caused what was thought to be an extremely exothermic dehydrohalogenation reaction that melted the neck of the flask, [Chem. Eng. News, 1981, 59(28), 4].Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.Профиль безопасности
Poison by ingestion and subcutaneous routes. Moderately toxic by intraperitoneal route. Questionable carcinogen with experimental carcinogenic data. Experimental reproductive effects. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of Cland Na2O. Used as an herbicide.Возможный контакт
This haloacetic acid can be a byproduct of drinking water disinfection and may increase the risk of cancer. Trichloroacetic acid is used as medication; in organic syntheses; as a reagent for albumin detection; as an intermediate in pesticide manufacture and in the production of sodium trichloroacetate which is itself a herbicide.Канцерогенность
TCA was not mutagenic in bacterial assays.The IARC has determined that there is limited evidence for the carcinogenicity of TCA in experimental animals and that it is not classifiable as to its carcinogenicity to humans. Neutralized TCA was not clastogenic in human lymphocytes in vitro or in the mouse bone marrow micronucleus test.Перевозки
UN1839 (solid) & UN2564 (solution) Trichloroacetic acid, solid and Trichloroacetic acid, solution, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Purify the acid by fractional crystallisation from its melt, then crystallise it repeatedly from dry *benzene and store it over conc H2SO4 in a vacuum desiccator. It can also be crystallised from CHCl3 or cyclohexane, and dried over P2O5 or Mg(ClO4)2 in a vacuum desiccator. Trichloroacetic acid can be fractionally distilled under reduced pressure from MgSO4. Layne, Jaffé and Zimmer [J Am Chem Soc 85 435 1963] dried trichloroacetic acid in *benzene by distilling off the *benzene-water azeotrope, then crystallised the acid from the remaining *benzene solution. Manipulations should be carried out under N2. [Toxic vapours, use a well ventilated fume cupboard.] [Beilstein 2 IV 508.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, silver salts, strong acids, strong bases, moisture, iron, zinc, aluminum. Corrosive to iron, steel and other metals.использованная литература
https://pubchem.ncbi.nlm.nih.gov/compound/trichloroacetic_acid#section=Tophttps://en.wikipedia.org/wiki/Trichloroacetic_acid
Трихлоруксусная кислота запасные части и сырье
Трихлоруксусная кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | ||
| +8617653113209 | China | 3049 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | ||
| +86 18953170293 | China | 2930 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |
Трихлоруксусная кислота Обзор)
1of4