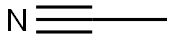
Ацетонитрил
- английское имяAcetonitrile
- CAS №75-05-8
- CBNumberCB2127174
- ФормулаC2H3N
- мольный вес41.05
- EINECS200-835-2
- номер MDLMFCD00001878
- файл Mol75-05-8.mol
| Температура плавления | ?45 °C (lit.) |
| Температура кипения | 81-82 °C (lit.) |
| плотность | 0.786 g/mL at 25 °C (lit.) |
| плотность пара | 1.41 (vs air) |
| давление пара | 72.8 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 48 °F |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | organic solvents: soluble(lit.) |
| пка | 25(at 25℃) |
| форма | liquid |
| цвет | <10(APHA) |
| Удельный вес | approximate 0.78(20/20℃) |
| Запах | Aromatic ether-like odor detectable at 40 ppm |
| Относительная полярность | 0.46 |
| Пределы взрываемости | 3.0-17%(V) |
| Порог?обнаружения?запаха? | 13ppm |
| Растворимость в воде | miscible |
| λмакс | λ: 195 nm Amax: ≤0.12 λ: 200 nm Amax: ≤0.032 λ: 230 nm Amax: ≤0.0044 λ: 235 nm Amax: ≤0.0044 λ: 250 nm Amax: ≤0.0044 λ: 400 nm Amax: ≤0.0044 |
| Мерк | 14,70 |
| БРН | 741857 |
| констант закона Генри | 7.30 at 5 °C, 8.90 at 10 °C, 11.6 at 15 °C, 14.6 at 20 °C, 17.6 at 25 °C (headspace-GC, Ji and Evans, 2007) |
| Пределы воздействия | TLV-TWA 70 mg/m3 (40 ppm) (ACGIH and OSHA); STEL 105 mg/m3 (60 ppm) (ACGIH); IDLH 4000 ppm (NIOSH). |
| Диэлектрическая постоянная | 37.5(21℃) |
| Стабильность | Incompatible with alkali metals, acids, bases, reducing agents and oxidizing agents. Highly flammable. |
| LogP | -0.340 |
| Surface tension | 28.95mN/m at 293.15K |
| FDA 21 CFR | 514.1 |
| Справочник по базе данных CAS | 75-05-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4-7 |
| FDA UNII | Z072SB282N |
| Справочник по химии NIST | Acetonitrile(75-05-8) |
| Система регистрации веществ EPA | Acetonitrile (75-05-8) |
| UNSPSC Code | 41116107 |
| NACRES | NB.21 |
| Коды опасности | F,Xi,Xn,T | |||||||||
| Заявления о рисках | 11-36-20/21/22-10-36/37/38-23/24/25-41-24-20/22 | |||||||||
| Заявления о безопасности | 16-36/37-45-36/37/39-27-26-36 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 20 ppm (34 mg/m3) | |||||||||
| РИДАДР | UN 1993 3/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | AL7700000 | |||||||||
| F | 9 | |||||||||
| Температура самовоспламенения | 524 °C | |||||||||
| Примечание об опасности | Highly Flammable/Harmful/Irritant | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29269095 | |||||||||
| Банк данных об опасных веществах | 75-05-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 3800 mg/kg (Smyth) | |||||||||
| ИДЛА | 137 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H302+H312+H332:Вредно при проглатывании, при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Ацетонитрил химические свойства, назначение, производство
Описание
Acetonitrile is a liquid with an etherlike odor. It is a highly polar, volatile solvent used in many different industrial applications. It is widely used in the pharmaceutical, photographic, chemical, and analytical industries. It is useful as an industrial solvent for the separation of olefins, polymers, spinning fibers, and plastics. Other uses include the extraction and refining of copper and by-product ammonium sulfate; used for dyeing textiles and in coating compositions; used as a stabilizer for chlorinated solvents; manufacture of perfumes and cosmetics; and as a general reagent in a wide variety of chemical processes.Химические свойства
Acetonitrile (methyl cyanide), CH3CN, is a colorless liquid with a sweet, ethereal odor. It is completely miscible with water and its high dielectric strength and dipole moment make it an excellent solvent for both inorganic and organic compounds including polymers. it is commonly applied to the development and manufacturing of cosmetics, pharmaceutical and agricultural products.Acetonitrile has been banned in cosmetic products in the European Economic Area (EEA) since early 2000 and acetone and ethyl are often preferred as safer for domestic use.Физические свойства
Colorless liquid with an ether-like or pungent odor of vinegar. A detection odor threshold concentration of 1,950 mg/m3 (1,161 ppmv) was experimentally determined by Dravnieks (1974). An odor threshold concentration of 13 ppmv was reported by Nagata and Takeuchi (1990).Использование
Acetonitrile is the simplest organic nitrile. It is a by-product of the manufacture of acrylonitrile, and acetonitrile has, in fact, replaced acrylonitrile. Acetonitrile has a number of uses, primarily as an extraction solvent for butadiene; as a chemical interme- diate in pesticide manufacturing; as a solvent for both inorganic and organic compounds; to remove tars, phenols, and coloring matter from petroleum hydrocarbons not soluble in acetonitrile; in the production of acrylic fi bers; in pharmaceuticals, perfumes, nitrile rubber, and acrylonitrile-butadiene-styrene (ABS) resins; in high-performance liquid and gas chro- matographic analysis; and in extraction and refi ning of copper. It is used as a starting material for the produc- tion of acetophenone, alpha-naphthalenacetic acid, thiamine, and acetamidine.прикладной
Acetonitrile is used as a solvent for polymers, spinning fibers, casting and molding plastics, and HPLC analyses; for extraction of butadiene and other olefins from hydrocarbon streams; in dyeing and coating textiles; and as a stabilizer for chlorinated solvents. It occurs in coal tar and forms as a by-product when acrylonitrile is made. Although acetonitrile is one of the more stable nitriles, it undergoes typical nitrile reactions and is used to produce many types of nitrogencontaining compounds.Acetonitrile also is used as a catalyst and as an ingredient in transitionmetal complex catalysts.Определение
ChEBI: Acetonitrile is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by a methyl group. It has a role as a polar aprotic solvent and an EC 3.5.1.4 (amidase) inhibitor. It is an aliphatic nitrile and a volatile organic compound.Методы производства
Acetonitrile is mainly prepared by dehydration of acetamide (CH3CONH2) with glacial acetic acid (Turner 1950) or by reacting acetic acid with ammonia at 400-500°C in the presence of a dehydration catalyst (Anon 1978).Общее описание
A colorless limpid liquid with an aromatic odor. Flash point 42°F. Density 0.783 c / cm3. Toxic by skin absorption. Less dense than water. Vapors are denser than air.Реакции воздуха и воды
Highly flammable. Water soluble.Профиль реактивности
Acetonitrile decomposes when heated to produce deadly toxic hydrogen cyanide gas and oxides of nitrogen. Strongly reactive [Hawley]. May react vigorously with strong oxidizing reagents, sulfuric acid, chlorosulfonic acid, sulfur trioxide, perchlorates, nitrating reagents, and nitric acid. [Sax, 9th ed., 1996, p. 20]. Potentially explosive in contact with nitrogen-fluorine compounds (e.g., tetrafluorourea) [Fraser, G. W. et al., Chem. Comm., 1966, p. 532].Угроза здоровью
Acetonitrile liquid or vapor is irritating to the skin, eyes, and respiratory tract. Acetonitrile has only a modest toxicity, but it can be metabolized in the body to hydrogen cyanide and thiocyanate. Acetonitrile causes delayed symptoms of poisoning (several hours after the exposure) that include, but are not limited to, salivation, nausea, vomiting, anxiety, confusion, hyperpnea, dyspnea, respiratory distress, disturbed pulse rate, unconscious- ness, convulsions, and coma. Cases of acetonitrile poisoning in humans (or, more strictly, of cyanide poisoning after exposure to acetonitrile) are rare but not unknown, by inha- lation, ingestion, and (possibly) by skin absorption. Repeated exposure to acetonitrile may cause headache, anorexia, dizziness, weakness, and macular, papular, or vesicular dermatitis.Воспламеняемость и взрывоопасность
Acetonitrile is a flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Acetonitrile vapor forms explosive mixtures with air at concentrations of 4 to 16% (by volume).Hazardous gases produced in a fire include hydrogen cyanide, carbon monoxide, carbon dioxide, and oxides of nitrogen. Carbon dioxide or dry chemical extinguishers should be used for acetonitrile fires.
Промышленное использование
Acetonitrile is used as a solvent both in industry and in the laboratory, as a rodenticide, and in the denaturation of alcohol. Because of both its solvent properties and volatility, it is useful for extracting vegetable and animal oils and dissolving hydrocarbons, oils, and greases. Acetonitrile is used for the purification of acetylene and artificial textile fibers, and as an antioxidant for rubber (Dequidt et al 1974). It has also been used to extract herbicide residues from soils (Smith 1980), to remove tars and other compounds from petroleum hydrocarbons, and to extract fatty acids from vegetable and fish liver oil. Acetonitrile is now a standard solvent component in reversed-phase high-performance liquid chromatography. It is the starting point for the syntheses of a number of organic compounds such as carboxylic acids and various nitrogen derivatives (Smiley 1981).Профиль безопасности
Poison by ingestion and intraperitoneal routes. Moderately toxic by several routes. An experimental teratogen. Other experimental reproductive effects. A skin and severe eye irritant. Human systemic effects by ingestion: convulsions, nausea or vomiting, and metabolic acidosis. Human respiratory system effects by inhalation. Mutation data reported. Dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosion Hazard: See also CYANIDE and NITRILES. When heated to decomposition it emits highly toxic fumes of CNand NOx,. Potentially explosive reaction with lanthanide perchlorates and nitrogen-fluorine compounds. Exothermic reaction with sulfuric acid at 53°C. Will react with water, steam, acids to produce toxic and flammable vapors. Incompatible with oleum, chlorosulfonic acid, perchlorates, nitrating agents, inchum, dinitrogen tetraoxide, N-fluoro compounds (e.g., perfluorourea + acetonitrile), HNO3, so3. To fight fire, use foam, Con, dry chemicalВозможный контакт
Acetonitrile is used as an extractant for animal and vegetable oils, as a solvent; particularly in the pharmaceutical industry, and as a chemical intermediate in pesticide manufacture; making batteries and rubber products. It is present in cigarette smokeОписание
Ацетонитрил (нитрил уксусной кислоты, этаннитрил, метилцианид) — органическое химическое соединение с формулой CH3CN. Представляет собой бесцветную жидкость со слабым эфирным запахом. Широко используется в органической химии в качестве растворителя.
Химические свойства
Ацетонитрил используется для растворения масел, жиров, лаков, эфиров целлюлозы, различных синтетических полимеров и неорганических солей.
Канцерогенность
Under the conditions of these 2- year inhalation studies by NTP, there was equivocal evidence of carcinogenic activity of acetonitrile in male F344/N rats based on marginally increased incidences of hepatocellular adenoma and carcinoma. There was no evidence of carcinogenic activity of acetonitrile in female F344/N rats exposed to 100, 200, or 400 ppm. There was no evidence of carcinogenic activity of acetonitrile in male or female B6C3F1 mice exposed to 50, 100, or 200 ppm. Exposure to acetonitrile by inhalation resulted in increased incidences of hepatic basophilic foci in male rats and of squamous hyperplasia of the forestomach in male and female mice.Применение
Ацетонитрил применяется как экстрагент для выделения бутадиена из
смеси углеводородов, азеотропный агент для выделения толуола, сырьё для
фармацевтической промышленности, растворитель.
Применяется также в биохимии как растворитель — важный компонент
подвижной фазы для анализа липидного состава биологических образцов (их
общелипидных экстрактов) методом высокоэффективной гидрофильной (Hilic)
хроматографии.
Метаболизм
Acetonitrile metabolism in dogs was demonstrated by Lang (1894), who reported that about 20% of the nitrile administered was converted to thio-cyanate in the urine, while guinea pigs metabolized acetonitrile to a greater extent (50% of dose excreted as thiocyanate). When the animals were pre-treated with ethanol, acetonitrile metabolism was induced (Tanii and Hashimoto 1986). In rats, acetone was found to potentiate acetonitrile toxicity and elevate cyanide concentrations in the blood (Freeman and Hays 1985). Baumann et al (1933) found that rabbits injected with acetonitrile excreted 27-35% of the dose as thiocyanate, while in thyroidectomized rabbits, the excretion decreased significantly (3-5% of the dose). Thiocyanate excretion was increased notably upon feeding dessicated thyroid to these animals. Hunt (1923) found that powdered sheep thyroid protected mice against acetonitrile toxicity. However, the role played by the thyroid in the detoxication of cyanide to thiocyanate is unclear. It has been suggested that the thyroid may have a role in the microsomal cleavage of cyanide from acetonitrile other than its direct effect on sulphation of cyanide to thiocyanate.Безопасность
Ацетонитрил токсичен, всасывается через кожу. В концентрации 15% или более является прекурсором.
хранилище
Acetonitrile should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.Производство
В промышленности ацетонитрил получают реакцией уксусной кислоты с
небольшим избытком аммиака при 300—400 °C в присутствии катализатора.
Выход в данном процессе составляет 90—95 %. Ацетонитрил образуется
также как побочный продукт в синтезе акрилонитрила при окислительном
аммонолизе пропилена.
В лабораторных условиях для синтеза ацетонитрила удобно применять
реакцию дегидратации ацетамида под действием пентаоксида фосфора.
Перевозки
UN1648 Acetonitrile, Hazard Class: 3; Labels: 3-Flammable liquidНесовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, chlorosulfonic acid, oleum, epoxides. May accumulate static electrical charges, and may cause ignition of its vapors. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acidsУтилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with nitrogen oxide removal from effluent gases by scrubbers or incineratorsАцетонитрил запасные части и сырье
сырьё
1of3
запасной предмет
- 2-THENOYLACETONITRILE
- Цис-2-аминоциклогексанол гидрохлорид
- МЕТИЛ 3 - ((ПИРРОЛИДИН-1-YL) МЕТИЛ) БЕНЗОАТ
- 1,1'-бис(дифенилфосфино)ферроцен-палладий(II)дихлорид дихлорметан аддукт в компании Реарус
- 1-(1-TERT-BUTYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOL-4-YL)-2,2,2-TRIFLUOROETHANONE
- Бис(диизопропиламино)хлорфосфин
- 4-Methyl-3-nitroanisole
- 2-Амино-5-хлорбензонитрил
- (E)-METHYL 3-(4-BROMOPHENYL)ACRYLATE
- Demosan
- 2-пропансульфанил хлорид
- 2,2,2-TRIFLUORO-1-(3-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOL-4-YL)ETHANONE
- 2-гидрокси-5-бромпиридин
- 6-CHLORO-[1,2,4]TRIAZOLO[4,3-B]PYRIDAZINE
- 1-фенилимидазол
- 3-AMINO-1-PHENYL-PROPAN-1-OL
- 1-Нафталинсульфонилхлорид
- Хинолин-2-карбонитрил
- 6-(BOC-AMINO)-HEXYL BROMIDE
- Ацетонитрильный комплекс трифторида бора
- Палладий(II) ацетилацетонат
- Methyl 3-(morpholinomethyl)benzoate ,98%
- CIS-9-TETRADECENYL ACETATE
- 1-Boc-piperazine acetate
- Дихлор [1,2-бис (дифенилфосфино) этан] палладий (II)
- Dichloro(1,5-cyclooctadiene)palladium(II)
- 1-БЕНЗИЛ-2-ИМИДАЗОЛКАРБОНОВАЯ КИСЛОТА
- Бензоилацетонитрил
- Диметил acetylmethylphosphonate
- 4- (2-гидроксиэтокси) бензальдегид
1of8
Ацетонитрил поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-86-60956847 +8617302186009 |
China | 3 | 58 | ||
| United States | 57505 | 58 | |||
| United States | 52924 | 58 | |||
| +86-0371-55170693 +86-19937530512 |
China | 21626 | 55 | ||
| 19565631292 19565631292 |
China | 185 | 58 | ||
| +86-025-86655873 +8613962173137 |
China | 195 | 55 | ||
| +86-15790943001 +86-15790943001 |
China | 374 | 58 | ||
| +86-15531157085; +8615531157085 |
China | 8805 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12776 | 58 | ||
| +8617736087130 | China | 994 | 58 |
Ацетонитрил Обзор)
1of4