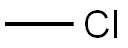
МЕТИЛХЛОРИД
- английское имяChloromethane
- CAS №74-87-3
- CBNumberCB9673584
- ФормулаCH3Cl
- мольный вес50.49
- EINECS200-817-4
- номер MDLMFCD00000872
- файл Mol74-87-3.mol
| Температура плавления | −97 °C(lit.) |
| Температура кипения | −24.2 °C(lit.) |
| плотность | 0.915 g/mL at 25 °C(lit.) |
| плотность пара | 1.74 (vs air) |
| давление пара | 3796 mm Hg ( 20 °C) |
| показатель преломления | 1.0007 |
| Fp | <-30 °F |
| температура хранения | 2-8°C |
| растворимость | water: soluble5.32g/L at 25°C |
| форма | Colorless gas |
| цвет | Colorless to Almost colorless |
| Запах | faint sweet ethereal odor |
| Пределы взрываемости | 19% |
| Растворимость в воде | 5.347g/L(24.9 ºC) |
| Мерк | 14,6041 |
| БРН | 1696839 |
| констант закона Генри | In seawater: 5.22 at 5 °C, 6.36 at 10 °C, 8.72 at 15 °C, 9.35 at 20 °C, 11.20 at 25 °C (Moore, 2000) |
| Пределы воздействия | TLV-TWA 50 ppm (~105 mg/m3) (ACGIH), 100 ppm (~210 mg/m3) (OSHA); ceiling 100 ppm (MSHA), 200 ppm (OSHA); TLV STEL 100 ppm (ACGIH); carcinogenicity: Animal Inadequate Evidence, Human Inad equate Evidence (IARC). |
| Диэлектрическая постоянная | 12.6(-20℃) |
| Стабильность | Stable. May react violently or explosively with interhalogens, magnesium, zinc, potassium, sodium or their alloys. Incompatible with natural rubber and neoprene composites, but does not attack PVA. Highly flammable. May decompose upon exposure to moist air or water. |
| Справочник по базе данных CAS | 74-87-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 6 |
| FDA UNII | A6R43525YO |
| Предложение 65 Список | Methyl Chloride |
| МАИР | 3 (Vol. 41, Sup 7, 71) 1999 |
| Система регистрации веществ EPA | Chloromethane (74-87-3) |
| UNSPSC Code | 12000000 |
| Коды опасности | F+,Xn,T,F | |||||||||
| Заявления о рисках | 12-40-48/20-67-66-22-19-38-23/25-11-39/23/24/25-23/24/25-62-63 | |||||||||
| Заявления о безопасности | 9-16-33-29-36-24-45-7-36/37 | |||||||||
| РИДАДР | UN 1993 3/PG 1 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | PA6300000 | |||||||||
| Температура самовоспламенения | 1169 °F | |||||||||
| кода HS | 2903.11.0010 | |||||||||
| Классификация DOT | 2.1 (Flammable gas) | |||||||||
| Класс опасности | 2.1 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 74-87-3(Hazardous Substances Data) | |||||||||
| Токсичность | LC50 (inhalation) for mice 3,146 ppm/7-h, rats 152,000 mg/m3/30-min (quoted, RTECS, 1985). | |||||||||
| ИДЛА | 2,000 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
H361fd:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению. Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
H420:Разрушает озоновый слой.
H221:Воспламеняющийся газ.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
P502:Обратиться к изготовителю или поставщику для получения информации о вторичной переработке или утилизации.
МЕТИЛХЛОРИД химические свойства, назначение, производство
Описание
Methyl chloride is a colorless, flammable gas with a faintly sweet, nonirritating odor at room temperature. It is shipped as a transparent liquid under its vapor pressure of about 59 psig at 70°F (407 kPa at 21.1℃).Methyl chloride burns feebly in air, but forms mixtures with air that can be explosive within its flammability range.
Dry methyl chloride is very stable at normal temperatures and in contact with air. In the presence of moisture, it hydrolyzes slowly, which results in the formation of corrosive hydrochloric acid. At temperatures above 700°F (371℃), methyl chloride may decompose into toxic end-products (hydrochloric acid, phosgene, chlorine, and carbon monoxide). It is slightly soluble in water and very soluble in alcohol, mineral oils, chloroform, and most organic liquids.
Химические свойства
Methyl chloride,CH3CI, is a flammable, narcotic,colorless compressed gas or liquid with a faintly sweet odor.Slightly soluble in water and soluble in alcohol this gas boils at -23.7℃ and freezes at -97.6℃ and is used as a refrigerant, catalyst carrier, and methylating agent. Also known as chloromethane.Физические свойства
Colorless, liquefied compressed gas, with a sweet, ethereal odor. Volatile flammable gas. An experimentally determined odor threshold concentration of >100 ppmv was reported by Leonardos et al. (1969).Использование
Methyl chloride is used as a refrigerant,as a local anesthetic, as a blowing agentfor polystyrene foams, and as a methylat ing agent in the synthesis of a number ofchemicals of commercial application.Методы производства
Methyl chloride has been used in rubber adhesives and other rubber solutions; in the pharmaceutical industry; as a paint and varnish remover; in solvent degreasing; in aerosol 2 JON B. REID AND CUSTODIO V. MUIANGA formulations; in food and drug processing; in the plastics industry; in hair sprays, insecticides, and spray paints; as a cosolvent or vapor pressure depressant; as a blowing agent for flexible polyurethane foams; as a cleaning solvent for printed circuit boards; as a stripper solvent for photoresists; as a solvent for cellulose acetate fiber; in plastic film; in protective coatings; in chemical processing; as a carrier solvent for herbicides and insecticides; to extract heatsensitive, naturally occurring substances such as cocoa, edible fats, spices, and beer hops; for decaffeinating coffee; as a refrigerant; in oil dewaxing; as a dye and perfume intermediate; in the textile industry; as a postharvest fumigant for strawberries; as a grain fumigant; for degreening citrus fruits; as an industrial solvent; in low-temperature extraction; as a solvent for oil, fats, bitumen, esters, resins, and rubber; in coating photographic films; as a food additive; in synthetic fibers and leather coatings; as a spotting agent; and in organic synthesis.Определение
ChEBI: A one-carbon compound that is methane in which one of the hydrogens is replaced by a chloro group.Общее описание
A colorless gas with a faint sweet odor. Shipped as a liquid under its vapor pressure. A leak may either be liquid or vapor. Contact with the liquid may cause frostbite by evaporative cooling. Easily ignited. Vapors heavier than air. Can asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. Used to make other chemicals and as a herbicide.Реакции воздуха и воды
Highly flammable.Профиль реактивности
METHYL CHLORIDE can react vigorously with oxidizing agents. May react explosively with sodium, potassium, sodium-potassium alloy, magnesium, zinc. Reacts with aluminum powder in the presence of catalytic amounts of aluminum chloride to form pyrophoric trimethylaluminum. When heated to decomposition, METHYL CHLORIDE emits highly toxic fumes of chlorine [Bretherick, 5th ed., 1995, p. 176].Опасность
Flammable, dangerous fire risk, explosive limits in air 10.7–17%. Narcotic. Psychic effects. Central nervous system impairment; liver, kidney and testicular damage, and teratogenic effects. Questionable carcinogen.Угроза здоровью
Inhalation of methyl chloride can produceheadache, dizziness, drowsiness, nausea,vomiting, convulsions, coma, and respiratoryfailure. It is narcotic at high concentrations.Repeated exposures can produce liver and Methyl chloride caused adverse reproduc tive effects in test animals. These includeembryo toxicity, fetal death, developmentalabnormalities, and paternal effects in rats andmice. It tested positive to the histidine rever sion–Ames test for mutagenicity. The car cinogenic properties of this compound havenot been established. The evidence in ani mals and humans is inadequate.Пожароопасность
Flammable gas, burns with a smoky flame; autoignition temperature 632°C (1170°F). Methyl chloride forms explosive mixtures with air within the range 7.6–19.0% by volume in air. It reacts explosively with alkali metals, potassium, sodium, or lithium; sodium–potassium alloy; and with magnesium, aluminum, or zinc in powder form.Использование в материалах
Dry methyl chloride may be contained in such common metals as steel, iron, copper, and bronze, but it has a corrosive action on zinc, aluminum, die castings, and possibly magnesium alloys. Methyl chloride must not be used with aluminum, since in the presence of moisture it forms spontaneously flammable methyl aluminum compounds upon contact with that metal. No reaction occurs, however, with the drying agent, activated alumina.Gaskets made of natural rubber and many neoprene compositions should be avoided because methyl chloride dissolves many organic materials. Pressed fiber gaskets, including those made of asbestos may be used with methyl chloride. Polyvinyl alcohol is unaffected by methyl chloride, and its use is also recommended. Medium- soft metal gaskets may be used for applications where alternating stresses such as those resulting from large temperature changes do not lead to "ironing out" and consequent leakage.
Возможный контакт
Methyl chloride is used as a methylating and chlorinating agent in organic chemistry; Used in production of silicones and tetramethyl lead. In petroleum refineries it is used as an extractant for greases, oils, and resins. Methyl chloride is also used as a solvent in the synthetic rubber industry; as a refrigerant; and as a propellant in polystyrene foam production. In the past it has been used as a local anesthetic (freezing). It is an intermediate in drug manufacture.Канцерогенность
Methyl chloride was mutagenic to bacteria and genotoxic in a number of mammalian cell systems in vitro.14 It gave positive results in the dominant lethal test in rats in vivo.NIOSH recommends that methyl chloride be considered a potential occupational teratogen and carcinogen.
The IARC states that there is inadequate evidence for the carcinogenicity of methyl chloride to experimental animals and humans.
Экологическая судьба
Biological. Enzymatic degradation of methyl chloride yielded formaldehyde (Vogel et al., 1987).Photolytic. Reported photooxidation products via OH radicals include formyl chloride, carbon monoxide, hydrogen chloride, and phosgene (Spence et al., 1976). In the presence of water, formyl chloride hydrolyzes to HCl and carbon monoxide, whereas phosgene hydrolyzes to hydrogen chloride and carbon monoxide (Morrison and Boyd, 1971).
Methyl chloride reacts with OH radicals in the atmosphere at a rate of 8.5 x 10-14 cm3/sec with a lifetime of 135 d (Cox et al., 1976).
Chemical/Physical. The estimated hydrolysis half-life at 25 °C and pH 7 is 0.93 yr (Mabey and Mill, 1978).
The evaporation half-life of methyl chloride (1 mg/L) from water at 25 °C using a shallow-pitch propeller stirrer at 200 rpm at an average depth of 6.5 cm was 27.6 min (Dilling, 1977).
хранилище
All personnel handling methyl chloride cylinders should be fully informed about the dangers that can arise from improper handling of methyl chloride. The cylinder and system should be grounded before use. Before introducing methyl chloride into any apparatus or equipment, it should be tested for leaks, all leaks repaired, and the apparatus thoroughly dried. Only nonsparking tools should be used with methyl chloride. Chemical safety goggles and/or a full-face shield should be used when handling liquid methyl chloride.Перевозки
UN1063 Methyl chloride, or Refrigerant gas R 40, Hazard Class: 2.1; Labels: 2.1-Flammable gas. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the ownerМетоды очистки
Bubble methyl chloride through a sintered-glass disc dipped into conc H2SO4, then wash it with water, condense it at low temperature and fractionally distil it. It has been distilled from AlCl3 at -80o. Alternatively, pass it through towers containing AlCl3, soda-lime and P2O5, then condense and fractionally distil it. Store it as a gas. [Beilstein 1 IV 28.]Несовместимости
Violent reaction with chemically active metals, such as potassium, powdered aluminum; zinc, and magnesium. Reaction with aluminum trichloride, ethylene. Reacts with water (hydrolyzes) to form hydrochloric acid. Attacks many metals in the presence of moistureУтилизация отходов
Return refillable compressed gas cylinders to supplier. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration with adequate scrubbing and ash disposal facilitiesМЕТИЛХЛОРИД запасные части и сырье
сырьё
1of2
запасной предмет
- 2-амино-5-метилпиридина
- coconutaminium trimethyl chloride
- Faropenem sodium hemipentahydrate
- Fluotrimazol
- хлорид н-Octadecyltrimethylammonium
- 4, 4-диметилизоксазолидин-3-он
- Гидроксипропил метил целлюлоза
- Polyethylene glycol dimethyl ether
- Метилцеллюлоза
- Дихлорметилсилана
1of7
МЕТИЛХЛОРИД поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-0519-85551759 +8613506123987 |
China | 8840 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 |