ТРИМЕТИЛХЛОРСИЛАН
- английское имяChlorotrimethylsilane
- CAS №75-77-4
- CBNumberCB4375627
- ФормулаC3H9ClSi
- мольный вес108.64
- EINECS200-900-5
- номер MDLMFCD00000502
- файл Mol75-77-4.mol
| Температура плавления | −40 °C(lit.) |
| Температура кипения | 57 °C(lit.) |
| плотность | 0.857 g/mL at 25 °C |
| плотность пара | 3.7 (vs air) |
| давление пара | 100 mm Hg ( 25 °C) |
| показатель преломления | n |
| Fp | 104 °F |
| температура хранения | Store below +30°C. |
| растворимость | Miscible with ether, benzene, diethylether and perchloroethylene. |
| форма | Liquid |
| Удельный вес | 0.8536 (27℃) |
| цвет | Clear colorless |
| Пределы взрываемости | 1.5-46%(V) |
| Растворимость в воде | REACTS |
| Гидролитическая чувствительность | 8: reacts rapidly with moisture, water, protic solvents |
| Чувствительный | Moisture Sensitive |
| БРН | 1209232 |
| Стабильность | Stable. Highly flammable - note low flash point. Reacts violently with water. Incompatible with water, moisture, strong oxidizing agents, strong acids, strong bases, aldehydes, alcohols, amines, esters, ketones. |
| ИнЧИКей | IJOOHPMOJXWVHK-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 75-77-4(CAS DataBase Reference) |
| FDA UNII | 62UO4690X6 |
| Справочник по химии NIST | Silane, chlorotrimethyl-(75-77-4) |
| Система регистрации веществ EPA | Trimethylchlorosilane (75-77-4) |
| UNSPSC Code | 12352302 |
| NACRES | NA.22 |
| Коды опасности | T,F,C,Xn | |||||||||
| Заявления о рисках | 20/21-36/38-34-21-14-11-37-35-19-40-10-67-20/21/22-52 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-16-36/37-7/9 | |||||||||
| РИДАДР | UN 2924 3/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | VV2710000 | |||||||||
| F | 10-21 | |||||||||
| Температура самовоспламенения | 752 °F | |||||||||
| Примечание об опасности | Highly Flammable/Corrosive/Moisture Sensitive | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29310095 | |||||||||
| Банк данных об опасных веществах | 75-77-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 4868 mg/kg LD50 dermal Rabbit 1530 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H301+H331:Токсично при проглатывании или при вдыхании.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
ТРИМЕТИЛХЛОРСИЛАН химические свойства, назначение, производство
Химические свойства
Trimethylchlorosilane is a colorless fuming liquid with a pungent odor. Readily hydrolyzed with liberation of hydrogen chloride; soluble in benzene, ether and perchloroethylene. Chlorotrimethylsilane is a chloro-organosilane compound mainly used for silylation reactions.Реакции
Related to the epoxide ring opening, TMSCl also mediates some cyclopropane ring opening reactions. For example, treatment of 1-aceto-2,2-dimethylcyclopropane with TMSCl and sodium chloride in acetonitrile at 55°C for 24 h generated 5-chloro-5-methyl-2-hexanone in 84% yield (eq 39).When sodium iodide was employed to replace sodium chloride, iodotrimethylsilane generated in situ, and the reaction completed under more facile conditions (rt and 12 h). Interestingly, the dominant product is 5-iodo-4,4-dimethyl-2-pentanone (eq 40), arising from iodide attacking at the less hindered secondary methylene carbon, instead of the quaternary dimethylmethylene carbon.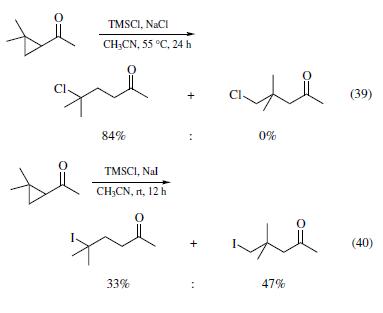
Использование
Chlorotrimethylsilane is a typical Silane Blocking Agent, which can protect or deprotect functional groups selectively. It have been used in the preparation of volatile derivatives of a wide range of compounds for GC analysis, and used for silylation and as a protection group in the process of various organic synthesis. It is used in the production of trimethylsilyl halides, pseudohalides and various organic silicon compounds. It is also used to produce hexamethyldisilane by reduction.Определение
ChEBI: Chlorotrimethylsilane is a silyl chloride consisting of a central silicon atom covalently bound to one chloro and three methyl groups. Chlorotrimethylsilane is a derivatisation agent used in gas chromatography/mass spectrometry applications. It has a role as a chromatographic reagent.Общее описание
Trimethylchlorosilane appears as a colorless fuming liquid with a pungent odor. Boiling point 135° F, Flash point -18°F. Density 0.854g/cm3. The vapor and liquid may cause burns. Vapors are heavier than air.Профиль реактивности
Chlorotrimethylsilane reacts vigorously and exothermically with water to produce hydrogen chloride.Угроза здоровью
Similar to other silanes. Toxicity is rated high for inhalation, ingestion and local irritation. May cause death or permanent injury after a very short exposure to small quantities.Пожароопасность
Violent reaction with water. Toxic and irritating hydrogen chloride and phosgene may be formed in fires. Difficult to extinguish, re-ignition may occur. Flashback along vapor trail may occur. Containers may explode in fire. Vapor may explode if ignited in enclosed area. When heated to decomposition or on contact with acids or acid fumes, chloride fumes are emitted. Reacts with surface moisture, releasing hydrogen chloride, which will corrode common metals and form flammable hydrogen gas. Avoid contact with water; Chlorotrimethylsilane readily hydrolyzes, liberating hydrochloric acid. Hazardous polymerization may not occur.Профиль безопасности
Poison by ingestion and skin contact. Moderately toxic by inhalation and intraperitoneal routes. A corrosive irritant to skin, eyes, and mucous membranes. Questionable carcinogen with experimental neoplastigenic data. Mutation data reported. A flammable liquid and very dangerous fire hazard when exposed to heat or flame. Violent reaction with water or hexafluoroisopropylideneamino lithium, A preparative hazard. To fight fire, use foam, alcohol foam, fog. When heated to decomposition it emits toxic fumes of Cl-. An intermediate in the production of silicones. See also CHLOROSILASES.Возможный контакт
Trimethylchlorosilane is used as an intermediate to make silicone products, including lubricantsПеревозки
UN1298 Trimethylchlorosilane, Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive material.Методы очистки
Likely impurities are other chlorinated methylsilanes and tetrachlorosilane (b 57.6o), some of which can form azeotropes. To avoid the latter, very efficient fractional distillation is required. It has been fractionated through a 12 plate glass helices-packed column with only the heart-cut material being used. It has also been fractionated through a 90cm, 19mm diameter Stedman column (p 11). Purify it by redistilling from CaH2 before use. [Sauer et al. J Am Chem Soc 70, 4254 1948, Sauer & Hadsell J Am Chem Soc 70 4258 1948, Langer et al. J Org Chem 23 50 1958, Beilstein 4 IV 4007.] FLAMMABLE and CORROSIVE.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Chlorosilanes react vigorously with bases and both organic and inorganic acids generating toxic and/or flammable gases. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous hydrogen. Attacks metals in the presence of moisture. Vigorous reaction with aluminum powder.Утилизация отходов
Do not discharge into drains or sewers. Use a licensed disposal contractor to an approved landfill. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office for guidance on acceptable disposal practices. The most favorable course of action is to use an alternative chemical product with less inherent propensity for occupational exposure or environmental contamination. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal, aquatic, and plant life; and conformance with environmental and public health regulations.ТРИМЕТИЛХЛОРСИЛАН запасные части и сырье
сырьё
1of2
запасной предмет
- 6-ЙОДПИРИДИН-2-КАРБОНОВАЯ КИСЛОТА
- Аллилтриметилсилана
- ETHYL 2,4-DIMETHYLQUINOLINE-3-CARBOXYLATE
- 5-Fluoro-2-picolinic acid
- BIS(TRIMETHYLSILYL)PEROXIDE
- (трифторметил)триметилсилан
- Рокитамицин
- ЭТИЛ-2-(ХЛОРМЕТИЛ)-4-МЕТИЛХИНОЛИН-3-КАРБОКСИЛАТ ГИДРОХЛОРИД
- 1-Метокси-3-триметилсилокси-1 ,3-бутадиен
- Тегафур
- Гексаметилдисилоксан
- (2R)-2-[(4-Ethyl-2,3-dioxopiperazinyl)carbonylamino]-2-phenylacetic acid
- 4-AMINO-2,6-DIFLUORO-BENZALDEHYDE
- Триметилсилилацетилен
- Азидотриметилсилан
- 4'-иодацетофенона
- ETHYL 2-(CHLOROMETHYL)-4-PHENYLQUINOLINE-3-CARBOXYLATE
- МЕТИЛ 2- (ХЛОРОМЕТИЛ) -4-МЕТИЛХИНОЛИН-3-КАРБОКСИЛАТ ГИДРОХЛОРИД
- (2R)-2-[(4-Ethyl-2,3-dioxopiperazinyl)carbonylamino]-2-(4-hydroxyphenyl)acetic acid
- Роданина-3-уксусной кислоты
- Бис (триметилсилил) ацетилен
- ETHYL 2-(CHLOROMETHYL)-4-PHENYLQUINOLINE-3-CARBOXYLATE HYDROCHLORIDE
- Цефоперазон
- Тиофен-2-этиламин
- Трис(триметилсилил)силан
- 6-Cyanonicotinic кислота
- 2- (ТРИМЕТИЛСИЛИЛ) ФЕНИЛ ТРИФЛУОРМЕТАНСУЛЬФОНАТ
- 5-БРОМ-3-ХЛОР-2-ПИРИДИНОН
- Анирацетам
- Thiazole-4-carboxaldehyde
1of8
ТРИМЕТИЛХЛОРСИЛАН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-371-66670886 | China | 19902 | 58 | |
| 0086-571-86990109 | China | 104 | 55 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 |
ТРИМЕТИЛХЛОРСИЛАН Обзор)
1of4