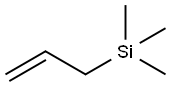
Аллилтриметилсилана
- английское имяAllyltrimethylsilane
- CAS №762-72-1
- CBNumberCB0431082
- ФормулаC6H14Si
- мольный вес114.26
- EINECS212-104-5
- номер MDLMFCD00008635
- файл Mol762-72-1.mol
химическое свойство
| Температура кипения | 84-88 °C (lit.) |
| плотность | 0.719 g/mL at 25 °C (lit.) |
| давление пара | 82hPa at 25℃ |
| показатель преломления | n |
| Fp | 45 °F |
| температура хранения | 2-8°C |
| растворимость | freely sol all organic solvents. |
| форма | Liquid |
| цвет | Clear colorless |
| Удельный вес | 0.72 |
| Растворимость в воде | insoluble |
| Гидролитическая чувствительность | 2: reacts with aqueous acid |
| БРН | 906755 |
| Стабильность | Volatile |
| ИнЧИКей | HYWCXWRMUZYRPH-UHFFFAOYSA-N |
| LogP | 4.64 at 20℃ |
| Справочник по базе данных CAS | 762-72-1(CAS DataBase Reference) |
| FDA UNII | 8B84C337VF |
| Справочник по химии NIST | Silane, trimethyl-2-propenyl-(762-72-1) |
| Система регистрации веществ EPA | Silane, trimethyl-2-propenyl- (762-72-1) |
| UNSPSC Code | 12352103 |
| NACRES | NA.22 |
больше
| Коды опасности | F,Xi | |||||||||
| Заявления о рисках | 11-36/37/38 | |||||||||
| Заявления о безопасности | 16-26-36-23-33-7/9 | |||||||||
| РИДАДР | UN 1993 3/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29310095 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
Аллилтриметилсилана химические свойства, назначение, производство
Химические свойства
clear colourless liquidИспользование
Allyltrimethylsilane is a general reagent to introduce allyl groups across acid chlorides, aldehydes, ketones, iminium ions, enones, and for cross-coupling with other carbon electrophiles. It is used as a reagent in Hosomi?Sakurai reaction.Подготовка
Allyltrimethylsilane is synthesized by the reaction of trimethylchlorosilane and allylmagnesium bromide.прикладной
Allyltrimethylsilane is used in the allylation of aldehydes, imines, allylic and benzylic alcohols, and chiral α-keto-amides that are derived from (S)-proline esters.Реакции
Allyltrimethylsilane is involved as a reactant in Hosomi Sakurai reaction for allylation in the presence of Lewis acid. For example, it reacts with cyclohexanone to get 1-allylcyclohexanol. It acts as a nucleophile and is involved in Carbon-Ferrier rearrangement.As a Carbon Nucleophile in Lewis Acid-Catalyzed Reactions.
Allyltrimethylsilane is an alkene some 10 times more nucleophilic than propene, as judged by its reactions with diarylmethyl cations.It reacts with a variety of cationic carbon electrophiles, usually prepared by coordination of a Lewis acid to a functional group, but also by chemical or electrochemical oxidation,or by irradiation in the presence of 9,10- dicyanoanthracene.
carbon to give an intermediate cation, and the silyl group is lost to create a double bond at the other terminus. Among the more straightforward electrophiles are acid chlorides (eq 1).
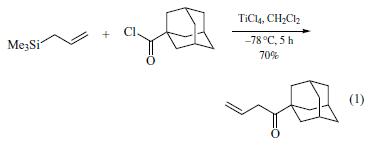
As a Carbon Nucleophile in Fluoride Ion-Catalyzed Reactions.
The reactions with aldehydes, ketones (eq 23),54 and α,β-unsaturated esters (eq 24)55 can also be catalyzed by fluoride ion, usually introduced as tetra-n-butylammonium fluoride (TBAF), or other silicophilic ions such as alkoxide. These reactions produce silyl ether intermediates, which are usually hydrolyzed before workup. The stereochemistry of attack on chiral ketones can sometimes be different for the Lewis acid- and fluoride ion-catalyzed reactions.
Other Reactions.
Allyltrimethylsilane reacts with some highly electrophilic alkenes, carbonyl compounds, azo compounds, and singlet oxygen to a greater or lesser extent in ene reactions that do not involve the loss of the silyl group, and hence give vinylsilanes in a solvent-dependent reaction.
Синтез
Preparation of allyl magnesium bromide Grignard reagent: Put magnesium and anhydrous ether into a 100OL reactor, heat to about 34°C, use a metering tank to pump a certain amount of 3-bromopropylene into the reactor, control the temperature at about 54°C and react for 4 hours. Preparation of allyl trimethylsilane: After the previous reaction, pump a certain amount of trimethyl chlorosilane into the reactor, control the temperature at about 54°C and react for 4 hours. Then, atmospheric distillation, and the distillate above 45°C, is the pure product.Методы очистки
Fractionate it through an efficient column at atmospheric pressure. If impure, dissolve it in THF, shake it with H2O (2x), dry (Na2SO4), filter and fractionate it. [Cudlin & Chvalovsky′ Collect Czech Chem Commun 27 1658 1962, Beilstein 4 IV 3927.]Аллилтриметилсилана запасные части и сырье
Аллилтриметилсилана поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined13337835376 | China | 40 | 58 | ||
| United States | 24072 | 58 | |||
| +862156762820 +86-13564624040 |
China | 7707 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +86-02784396550 +86-17721064038 |
China | 415 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 |