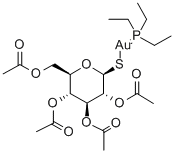
Ауранофин
- английское имяAuranofin
- CAS №34031-32-8
- CBNumberCB5363410
- ФормулаC20H34AuO9PS
- мольный вес678.48
- EINECS251-801-9
- номер MDLMFCD00080759
- файл Mol34031-32-8.mol
| Температура плавления | 112-115° |
| температура хранения | room temp |
| растворимость | DMSO: ≥5mg/mL |
| форма | solid |
| цвет | Crystals |
| Стабильность | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| FDA UNII | 3H04W2810V |
| Предложение 65 Список | Auranofin |
| Словарь наркотиков NCI | auranofin |
| Код УВД | M01CB03 |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 63-22 | |||||||||
| Заявления о безопасности | 36/37 | |||||||||
| РИДАДР | UN 2811 6.1 / PGIII | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | MD6500000 | |||||||||
| Банк данных об опасных веществах | 34031-32-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rats, mice (mg/kg): 265, 310 orally (Payne, Walz) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H361:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
Ауранофин химические свойства, назначение, производство
Описание
Auranofin is the first orally effective gold compound to be marketed for the treatment of severe rheumatoid arthritis. It is better tolerated and more convenient than gold sodium thiomalate, which is administered intramuscularly.Химические свойства
White to Off-White SolidИспользование
Auranofin is a new oral gold-based antiarthritis drug. Auranofin inhibits various leukocyte activation pathways at multiple sites. Auranofin inhibits the release of inflammatory mediators from human m acrophages, basophils, and pulmonary mast cells. Auranofin is an efficient inducer of mitochondrial membrane permeability transition pore in the presence of calcium ions related to its inhibition of m itochondrial thioredoxin reductase.Определение
ChEBI: An S-glycosyl compound consisting of 2,3,4,6-tetra-O-acetyl-1-thio-beta-D-glucopyranose with the sufur atom coordinated to (triethylphosphoranylidene)gold. It is administered orally fo the treatment of active progressive rheumatoid arthritis.Фармацевтические приложения
Auranofin ([tetra-O-acetyl-β-D- (glucopyranosyl)thio]-triethylphosphine)gold(I) is a second-generation gold-based drug, licensed as an orally available gold drug for the treatment of RA. It features a linear S Au P geometry, as shown by X-ray analysis. It is more lipophilic than the first-generation drugs, which makes oral administration possible. Treatment with Auranofin requires less visits to the clinic, but it is believed to be less successful in the treatment of RA compared to gold drugs being administered intramuscularly.Клиническое использование
Auranofin is indicated in adults with active rheumatoid arthritis who have not responded sufficiently to one or more NSAIDs.Профиль безопасности
Poison by ingestion,intraperitoneal, and intravenous routes. Human systemiceffects by ingestion: ulceration or bleeding from stomach.An experimental teratogen. Other experimentalreproductive effects. Human mutation data reported.When heated to decompСинтез
Synthesis: ethanolic thiodiglycol is treated first with aqueous gold(I) acid chloride trihydrate, then with ethanolic triethylphosphine to give triethylphosphine gold(I) chloride, which is added to an aqueous solution of S-(2,3,4,6-tetra-O-acetylglucopyranosyl)pseudothiourea hydrobromide and potassium carbonate to give the desired auranofin.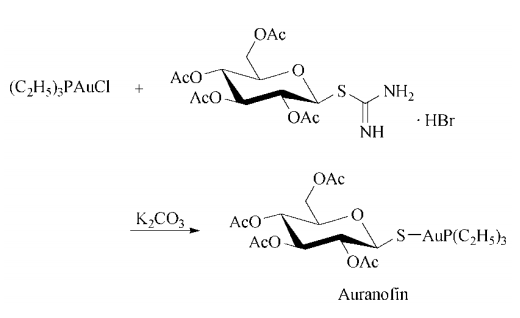
Метаболизм
On a mg gold/kg basis, it is reported to be as effective in the rat adjuvant arthritis assay as the parenterally effective drugs. Daily oral doses produce a rapid increase in kidney and blood gold levels for the first 3 days of treatment, with a more gradual increase on subsequent administration. Plasma gold levels are lower than those attained with parenteral gold compounds. The major route of excretion is via the urine. Auranofin may produce fewer adverse reactions than parenteral gold compounds, but its therapeutic efficacy also may be less.Ауранофин запасные части и сырье
Ауранофин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-371-66670886 | China | 19902 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +1-631-485-4226 | United States | 19553 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 |