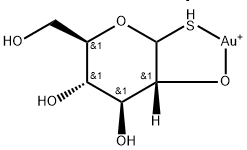
Ауротиоглюкоза
- английское имяAurothioglucose
- CAS №12192-57-3
- CBNumberCB4140009
- ФормулаC6H11AuO5S
- мольный вес392.18
- EINECS235-365-7
- номер MDLMFCD00056080
- файл Mol12192-57-3.mol
химическое свойство
| Температура плавления | >107oC (dec.) |
| температура хранения | 2-8°C |
| растворимость | H2O: soluble5mg/mL, clear |
| форма | powder |
| цвет | white to beige |
| Биологические источники | rabbit |
| Растворимость в воде | H2O: 5mg/mL, clear |
| Мерк | 13,887 |
| Стабильность | Hygroscopic, Light Sensitive |
| FDA UNII | 2P2V9Q0E78 |
| Код УВД | M01CB04 |
| МАИР | 3 (Vol. 13, Sup 7) 1987 |
| Система регистрации веществ EPA | Aurothioglucose (12192-57-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | Xn |
| Заявления о рисках | 42/43 |
| Заявления о безопасности | 22-36/37-45 |
| РИДАДР | 2811 |
| WGK Германия | 3 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| кода HS | 2843300000 |
| Банк данных об опасных веществах | 12192-57-3(Hazardous Substances Data) |
| Токсичность | LD50 intravenous in chicken: 1gm/kg |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
Ауротиоглюкоза химические свойства, назначение, производство
Описание
Aurothioglucose is highly water soluble, and its aqueous solutions decompose on long standing. It therefore is available as a suspension in sesame oil. Gold content is approximately 50%. Following IM injection, it is highly protein bound (95%), and peak plasma levels are achieved within 2 to 6 hours. Following a single 50-mg dose, the biological half-life ranges from 3 to 27 days, but following successive weekly doses, the half-life increases to 14 to 40 days after the third dose. The therapeutic effect does not correlate with serum plasma gold levels but appears to depend on total accumulated gold. Aurothioglucose is indicated for the adjunctive treatment of adult and juvenile rheumatoid arthritis.Химические свойства
yellow crystals,История
In 1927, aurothioglucose was found to relieve joint pain when used to treat bacterial endocarditis. The area of chrysotherapy had begun. Subsequent investigations led to an extensive study of gold compounds in Great Britain by the Empire Rheumatism Council, which reported in 1961 that sodium aurothiomalate was effective in slowing the development of progressive joint diseases. Both aurothioglucose and sodium aurothiomalate are orally ineffective and are administered by IM injection. In 1985, the first orally effective gold compound for arthritis, auranofin, was introduced in the United States. Several other gold compounds have been evaluated clinically but do not appear to offer advantages in terms of efficacy or toxicity.Использование
Aurothioglucose hydrate has been used in redox stress survival assay in human cell lines and for inducing obese phenotype in mice.Клиническое использование
Aurothioglucose is highly water soluble, and its aqueous solutions decompose on long standing. It therefore is available as a suspension in sesame oil. Gold content is approximately 50%. Following IM injection, it is highly protein bound (95%), and peak plasma levels are achieved within 2 to 6 hours. Following a single 50-mg dose, the biological half-life ranges from 3 to 27 days, but following successive weekly doses, the half-life increases to 14 to 40 days after the third dose. The therapeutic effect does not correlate with serum plasma gold levels but appears to depend on total accumulated gold. Aurothioglucose is indicated for the adjunctive treatment of adult and juvenile rheumatoid arthritis.Профиль безопасности
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. A deadly human poison by an unspecified route. An experimental poison by intramuscular route. Moderately toxic by subcutaneous and intravenous routes. Human systemic effects: nausea or vomiting, cholestatic jaundlce, and eye effects. An experimental teratogen. Other experimental reproductive effects. See also GOLD COMPOUNDS. When heated to decomposition it emits very toxic fumes of SOx. Used to treat rheumatoid arthritis.Синтез
Synthesis: gold thioglucose is prepared by adding a solution of gold bromide to an aqueous solution of thioglucose that contains sulfur dioxide. After heating, the product is precipitated by the addition of ethanol.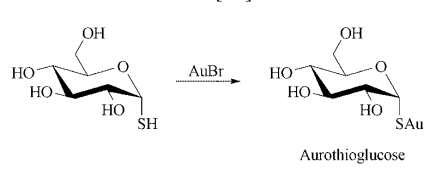
Методы очистки
Purify it by dissolving it in H2O (0.05g in 1mL) and precipitating it by adding EtOH. It yields yellow crystals with a slight mercaptan odour. It decomposes slowly in H2O, and is soluble in propylene glycol but insoluble in EtOH and other common organic solvents. [Caterson & Taylor FEBS Lett 98 351 1979, Cooney et al. Biochem J 259 651 1989.]Ауротиоглюкоза поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-852-30606658 | China | 43340 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | |
| +1-+1(833)-552-7181 | United States | 52924 | 58 | |
| +86-18186686046 +86-18186686046 |
China | 5861 | 58 | |
| +8613817748580 | China | 40066 | 58 | |
| +8613063595538 | China | 9363 | 58 |