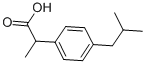
Ибупрофен
- английское имяIbuprofen
- CAS №15687-27-1
- CBNumberCB4336930
- ФормулаC13H18O2
- мольный вес206.28
- EINECS239-784-6
- номер MDLMFCD00010393
- файл Mol15687-27-1.mol
| Температура плавления | 77-78 °C |
| альфа | [α]D20 -1~+1°(c=1,C2H5OH) |
| Температура кипения | 157 °C (4 mmHg) |
| плотность | 1.0364 (rough estimate) |
| показатель преломления | 1.5500 (estimate) |
| Fp | 9℃ |
| температура хранения | 2-8°C |
| растворимость | Practically insoluble in water, freely soluble in acetone, in methanol and in methylene chloride. It dissolves in dilute solutions of alkali hydroxides and carbonates. |
| пка | pKa 4.45± 0.04(H2O,t = 25±0.5,I=0.15(KCl))(Approximate) |
| форма | Crystalline Powder |
| цвет | white to off-white |
| Растворимость в воде | insoluble |
| Мерк | 14,4881 |
| BCS Class | 2 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | HEFNNWSXXWATRW-UHFFFAOYSA-N |
| LogP | 3.970 |
| Справочник по базе данных CAS | 15687-27-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| Словарь онкологических терминов NCI | Advil; ibuprofen; Motrin |
| FDA UNII | WK2XYI10QM |
| Словарь наркотиков NCI | ibuprofen |
| Код УВД | C01EB16,G02CC01,M01AE01,M01AE51,M02AA13,R02AX02 |
| Справочник по химии NIST | Ibuprofen(15687-27-1) |
| Система регистрации веществ EPA | Benzeneacetic acid, .alpha.-methyl-4-(2-methylpropyl)- (15687-27-1) |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-63-51/53-39/23/24/25-23/24/25-11 | |||||||||
| Заявления о безопасности | 36-61-36/37-45-16-7 | |||||||||
| кода HS | 29163920 | |||||||||
| Банк данных об опасных веществах | 15687-27-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in male mice, rats (mg/kg): 495, 626 i.p.; 1255, 1050 orally (Orzalesi) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Ибупрофен химические свойства, назначение, производство
Химические свойства
Colourless, Crystalline SolidИстория
Ibuprofen was developed while searching for an alternative pain reliever to aspirin in the 1950s. It and related compounds were synthesized in 1961 by Stewart Adams, John Nicholson, and Colin Burrows who were working for the Boots Pure Drug Company in Great Britain. Adams and Nicholson filed for a British patent on ibuprofen in 1962 and obtained the patent in 1964; subsequent patents were obtained in the United States. The patent of Adams and Nicholson was for the invention of phenylalkane derivatives of the form shown in Figure 49.1, where R1 could be various alkyl groups, R2 was hydrogen or methyl, and X was COOH or COOR, with R being alkyl or aminoalkyl groups. The first clinical trials for ibuprofen were started in 1966. Ibuprofen was introduced under the trade name Brufen in 1969 in Great Britain. It was introduced in the United States in 1974. Ibuprofen was initially off ered by prescription, but it became available in over-the-counter medications in the 1980s.Использование
A common goal in the development of pain and inflammation medicines has been the creation of compounds that have the ability to treat inflammation, fever, and pain without disrupting other physiological functions. General pain relievers, such as aspirin and ibuprofen, inhibit both COX-1 and COX-2. A medication's specificaction toward COX-1 versus COX-2 determines the potential for adverse side effects. Medications with greater specificity toward COX-1 will have greater potential for producing adverse side effects. By deactivating COX-1, nonselective pain relievers increase the chance of undesirable side effects, especially digestive problems such as stomach ulcers and gastrointestinal bleeding. COX-2 inhibitors, such as Vioxx and Celebrex, selectively deactivate COX-2 and do not aff ect COX-1 at prescribed dosages. COX-2 inhibitors are widely prescribed for arthritis and pain relief. In 2004, the Food and Drug Administration (FDA) announced that an increased risk of heart attack and stroke was associated with certain COX-2 inhibitors. This led to warning labels and voluntary removal of products from the market by drug producers; for example, Merck took Vioxx off the market in 2004. Although ibuprofen inhibits both COX-1 and COX-2, it has several times the specificity toward COX-2 compared to aspirin, producing fewer gastrointestinal side effects.Показания
Ibuprofen (Advil, Motrin) is used as an analgesic and antipyretic as well as a treatment for rheumatoid arthritis and degenerative joint disease. The most frequently observed side effects are nausea, heartburn, epigastric pain, rash, and dizziness. Incidence of GI side effects is lower than with indomethacin.Visual changes and cross-sensitivity to aspirin have been reported. Ibuprofen inhibits COX-1 and COX-2 about equally. It decreases platelet aggregation, but the duration is shorter and the effect quantitatively lower than with aspirin. Ibuprofen prolongs bleeding times toward high normal value and should be used with caution in patients who have coagulation deficits or are receiving anticoagulant therapy.Определение
ChEBI: A monocarboxylic acid that is propionic acid in which one of the hydrogens at position 2 is substituted by a 4-(2-methylpropyl)phenyl group.Всемирная организация здравоохранения(ВОЗ)
Ibuprofen, a non-steroidal anti-inflammatory agent, was introduced in 1969. It was approved for sale without prescription in packages containing no more than 400 mg, in the United Kingdom in 1983. This action was followed by the USA, Canada and several European countries. Since this time reports of suspected adverse effects have increased. Most of these relate to gastrointestinal disturbances, hypersensitivity reactions but aseptic meningitis, skin rashes and renal damage have been recorded.Общее описание
Ibuprofen, 2-(4-isobutylphenyl)propionic acid (Motrin,Advil, Nuprin), was introduced into clinical practice followingextensive clinical trials. It appears to have comparableefficacy to aspirin in the treatment of RA, but with a lowerincidence of side effects. It has also been approved for usein the treatment of primary dysmenorrhea, which is thoughtto be caused by an excessive concentration of PGs and endoperoxides. However, a recent study indicates that concurrentuse of ibuprofen and aspirin may actually interferewith the cardioprotective effects of aspirin, at least in patientswith established cardiovascular disease. This is becauseibuprofen can reversibly bind to the platelet COX-1isozymes, thereby blocking aspirin’s ability to inhibit TXA2synthesis in platelets.Фармакокине?тика
Ibuprofen is rapidly absorbed on oral administration, with peak plasma levels being generally attained within 2 hours and a duration of action of less than 6 hours. As with most of these acidic NSAIDs, ibuprofen (pKa = 4.4) is extensively bound to plasma proteins (99%) and will interact with other acidic drugs that are protein bound.Клиническое использование
Ibuprofen is indicated for the relief of the signs and symptoms of rheumatoid arthritis and osteoarthritis, the relief of mild to moderate pain, the reduction of fever, and the treatment of dysmenorrhea.Экологическая судьба
Ibuprofen has a high water solubility and low volatility, which suggest a high mobility in the aquatic environment. This makes it a commonly detected chemical of the pharmaceutical and personal care products (PPCPs) in the environment. It is not as persistent, however, as many other chemicals. Ibuprofen undergoes photodegradation with exposure to direct and indirect sunlight, although degradation products can have effects on aquatic environments.Метаболизм
Metabolism occurs rapidly, and the drug is nearly completely excreted in the urine as unchanged drug and oxidative metabolites within 24 hours following administration. Metabolism by CYP2C9 (90%) and CYP2C19 (10%) involves primarily ω-, and ω1-, and ω2-oxidation of the p-isobutyl side chain, followed by alcohol oxidation of the primary alcohol resulting from ω–oxidation to the corresponding carboxylic acid. All metabolites are inactive. When ibuprofen is administered as the individual enantiomers, the major metabolite isolated is the S-(+)-enantiomer whatever the configuration of the starting enantiomer. Interestingly, the R-(–)-enantiomer is inverted to the S-(+)-enantiomer in vivo via an acetyl–coenzyme A intermediate, accounting for the observation that the two enantiomers are bioequivalent in vivo. This is a metabolic phenomenon that also has been observed for other arylpropionic acids, such as ketoprofen, benoxaprofen, fenoprofen, and naproxen.Ибупрофен запасные части и сырье
Ибупрофен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 0917-3909592 13892490616 |
China | 9316 | 58 | ||
| +8615271838296 | China | 1534 | 58 | ||
| +86-19930503282 | China | 8820 | 58 | ||
| +86-16631818819 +86-17736933208 |
China | 9311 | 58 | ||
| +8615350571055 | China | 5848 | 58 | ||
| +8617531190177 | China | 5873 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2503 | 58 | ||
| +86-86-18148706580 +8618826483838 |
China | 152 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10991 | 58 | ||
| +8617756083858 | China | 994 | 58 |