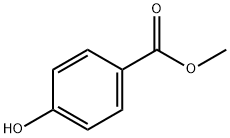
Метилпарабен
- английское имяMethylparaben
- CAS №99-76-3
- CBNumberCB0184566
- ФормулаC8H8O3
- мольный вес152.15
- EINECS202-785-7
- номер MDLMFCD00002352
- файл Mol99-76-3.mol
химическое свойство
| Температура плавления | 125-128 °C (lit.) |
| Температура кипения | 298.6 °C |
| плотность | 1,46g/cm |
| давление пара | 0.000005 hPa (20 °C) |
| показатель преломления | 1.4447 (estimate) |
| FEMA | 2710 | METHYL P-HYDROXYBENZOATE |
| Fp | 280°C |
| температура хранения | room temp |
| растворимость | ethanol: soluble0.1M, clear, colorless |
| пка | pKa 8.15(H2O,t =20.0) (Uncertain) |
| форма | Crystalline Powder |
| цвет | White to almost white |
| РН | 5.8 (H2O, 20°C) (saturated solution) |
| Запах | odorless or faint char. odor, sl. burning taste |
| Биологические источники | synthetic |
| Растворимость в воде | Slightly soluble in water. |
| Точка замерзания | 131℃ |
| Мерк | 14,6107 |
| БРН | 509801 |
| Стабильность | Stable. Incompatible with strong oxidizing agents, strong bases. |
| ИнЧИКей | LXCFILQKKLGQFO-UHFFFAOYSA-N |
| LogP | 1.98 at 20℃ |
| FDA 21 CFR | 184.1490; 582.3490; 172.515; 201.327; 310.545; 352.70 |
| Вещества, добавляемые в пищу (ранее EAFUS) | METHYL P-HYDROXYBENZOATE |
| Специальный комитет по веществам GRAS | Methyl Paraben |
| Справочник по базе данных CAS | 99-76-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4 |
| FDA UNII | A2I8C7HI9T |
| Справочник по химии NIST | Benzoic acid, 4-hydroxy-, methyl ester(99-76-3) |
| Система регистрации веществ EPA | Methylparaben (99-76-3) |
| UNSPSC Code | 51452201 |
| NACRES | NA.77 |
больше
| Коды опасности | Xi,Xn | |||||||||
| Заявления о рисках | 36/37/38-20/21/22-36 | |||||||||
| Заявления о безопасности | 26-36-24/25-39 | |||||||||
| РИДАДР | UN 2769 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | DH2450000 | |||||||||
| Температура самовоспламенения | >600 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29182930 | |||||||||
| Банк данных об опасных веществах | 99-76-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2100 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H411:Токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P391:Ликвидировать просыпания/проливы/утечки.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Метилпарабен химические свойства, назначение, производство
Химические свойства
Methylparaben occurs as colorless crystals or a white crystalline powder. It is odorless or almost odorless and has a slight burning taste. Soluble in alcohol,ether; slightly soluble in water, benzene, and carbontetrachloride.Вхождение
Reported present in cloudberry, yellow passion fruit juice, white wine, botrytised wine and Bourbon vanilla.Использование
Methylparaben and propylparaben are the most common of these. parabens is one of the most commonly used group of preservatives in the cosmetic, pharmaceutical, and food industries. Parabens provide bacteriostatic and fungistatic activity against a diverse number of organisms, and are considered safe for use in cosmetics, particularly in light of their low sensitizing potential. An evaluation of preservatives for use in leave-on cosmetic preparations lists parabens among the least sensitizing. The range of concentrations used in cosmetics varies between 0.03 and 0.30 percent, depending on the conditions for use and the product to which the paraben is added.Methylparaben is one of the most popular preservatives in beauty products and food items. According to the National Library of Medicine, the ingredient occurs naturally in a handful of fruits—like blueberries—though it can also be created synthetically.It's found in everything from cream cleansers and moisturizers to primers and foundations and helps these products maintain their effectiveness. Rabach says that it's chock-full of anti-fungal and antibacterial properties, which work wonders to extend the shelf life of skincare, haircare, and cosmetic products.
Определение
ChEBI: Methylparaben is a 4-hydroxybenzoate ester resulting from the formal condensation of the carboxy group of 4-hydroxybenzoic acid with methanol. It is the most frequently used antimicrobial preservative in cosmetics. It occurs naturally in several fruits, particularly in blueberries. It has a role as a plant metabolite, an antimicrobial food preservative, a neuroprotective agent and an antifungal agent.Подготовка
Methylparaben is produced through the methanol esterification of p-hydroxybenzoic acid in the presence of sulfuric acid. The materials are heated in a glass-lined reactor and distilled under reflux. The resulting acid is neutralized with caustic soda, then crystallized through cooling. The crystallized product is centrifuged, washed, dried under vacuum, milled and blended, all in corrosion-resistant equipment to avoid metallic contamination.Общее описание
Methyl paraben is basically a methyl ester of p-hydroxybenzoic acid. It is non-toxic, and non-carcinogenic in nature. It is a stable, non-volatile compound and finds application as an anti-microbial preservative in foods, drugs and cosmetics. It is readily absorbed through the skin and gastrointestinal tract. Upon hydrolyzation, it is hydrolyzed to p-hydroxybenzoic acid, and the conjugates formed get rapidly excreted in the urine.Опасность
Toxic. Use in foods restricted to 0.1%.Фармацевтические приложения
Methylparaben is widely used as an antimicrobial preservative in cosmetics, food products, and pharmaceutical formulations; see Table I. It may be used either alone or in combination with other methylparaben is the most frequently used antimicrobial preservative.The parabens are effective over a wide pH range and have a broad spectrum of antimicrobial activity, although they are most effective against yeasts and molds. Antimicrobial activity increases as the chain length of the alkyl moiety is increased, but aqueous solubility decreases; therefore a mixture of parabens is frequently used to provide effective preservation. Preservative efficacy is also improved by the addition of propylene glycol (2–5%), or by using parabens in combination with other antimicrobial agents such as imidurea;
Owing to the poor solubility of the parabens, paraben salts (particularly the sodium salt) are more frequently used in formulations. However, this raises the pH of poorly buffered formulations.
Methylparaben (0.18%) together with propylparaben (0.02%) has been used for the preservation of various parenteral pharmaceutical formulations;
Контактные аллергены
This substance is one of the parabens family. Parabens are esters formed by p-hydroxybenzoic acid and an alcohol. They are largely used as biocides in cosmetics and toiletries, medicaments, or food. They have synergistic power with other biocides. Parabens can induce allergic contact dermatitis, mainly in chronic dermatitis and wounded skin.Канцерогенность
The carcinogenic potential of methyl paraben has been studied in rodents. Several studies are available, but none that expose animals via oral or dermal routes. No evidence of a carcinogenic effect was observed following intravenous or subcutaneous injection .хранилище
Aqueous solutions of methylparaben at pH 3–6 may be sterilized by autoclaving at 120°C for 20 minutes, without decomposition. Aqueous solutions at pH 3–6 are stable (less than 10% decomposition) for up to about 4 years at room temperature, while aqueous solutions at pH 8 or above are subject to rapid hydrolysis (10% or more after about 60 days storage at room temperature);Methylparaben should be stored in a well-closed container in a cool, dry place.
Методы очистки
Fractionally crystallise the ester from its melt, and recrystallise it from *benzene, then from *benzene/MeOH and dry it over CaCl2 in a vacuum desiccator. [Beilstein 10 IV 360.]Несовместимости
The antimicrobial activity of methylparaben and other parabens is considerably reduced in the presence of nonionic surfactants, such as polysorbate 80, as a result of micellization.However, propylene glycol (10%) has been shown to potentiate the antimicrobial activity of the parabens in the presence of nonionic surfactants and prevents the interaction between methylparaben and polysorbate 80.Incompatibilities with other substances, such as bentonite, magnesium trisilicate,talc,tragacanth,sodium alginate, essential oils,sorbitol,and atropine,have been reported. It also reacts with various sugars and related sugar alcohols. Absorption of methylparaben by plastics has also been reported; the amount absorbed is dependent upon the type of plastic and the vehicle. It has been claimed that low-density and high-density polyethylene bottles do not absorb methylparaben.
Methylparaben is discolored in the presence of iron and is subject to hydrolysis by weak alkalis and strong acids.
Регуляторный статус
Methylparaben and propylparaben are affirmed GRAS Direct Food Substances in the USA at levels up to 0.1%. All esters except the benzyl ester are allowed for injection in Japan. In cosmetics, the EU and Brazil allow use of each paraben at 0.4%, but the total of all parabens may not exceed 0.8%. The upper limit in Japan is 1.0%.Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM, IV, and SC injections; inhalation preparations; ophthalmic preparations; oral capsules, tablets, solutions and suspensions; otic, rectal, topical, and vaginal preparations). Included in medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
использованная литература
The Protective Effect of Rosmarinic Acid against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts DOI:10.3390/nu12051282https://www.byrdie.com/methylparaben-for-skin-4779820
https://pubchem.ncbi.nlm.nih.gov/compound/Methylparaben
Handa O, et al. (2006). Methylparaben potentiates UV-induced damage of skin keratinocytes. DOI: 10.1016/j.tox.2006.07.018
Parabens in Cosmetics https://www.fda.gov/cosmetics/productsingredients/ingredients/ucm128042.htm
Okamoto T, et al. (2008). Combined activation of methyl paraben by light irradiation and metabolism toward oxidative DNA damage. DOI:10.1021/tx800066u
Метилпарабен запасные части и сырье
сырьё
1of2
запасной предмет
1of2
Метилпарабен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-570-3080488 +8615068935117 |
China | 1 | 58 | ||
| 510-85010237 | CHINA | 41 | 58 | ||
| +8615902132654 | China | 654 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +8613223293093 | China | 80 | 58 | ||
| +86-027-59207850 | China | 5972 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +8617732866630 | China | 18147 | 58 |
Метилпарабен Обзор)
1of4