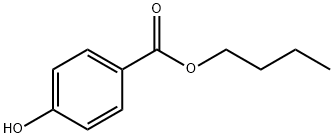
Бутилпарабен
- английское имяButylparaben
- CAS №94-26-8
- CBNumberCB5466788
- ФормулаC11H14O3
- мольный вес194.23
- EINECS202-318-7
- номер MDLMFCD00016478
- файл Mol94-26-8.mol
| Температура плавления | 67-70 °C(lit.) |
| Температура кипения | 156-157 °C3.5 mm Hg(lit.) |
| плотность | 1.28 |
| давление пара | 0.002-0.113Pa at 20-50℃ |
| FEMA | 2203 | BUTYL P-HYDROXY BENZOATE |
| показатель преломления | 1.5115 (estimate) |
| Fp | 181℃ |
| температура хранения | 2-8°C |
| растворимость | Soluble in DMSO, ethyl acetate, methanol. |
| форма | Crystalline Powder |
| пка | pKa 8.5 (Uncertain) |
| цвет | White to almost white |
| Запах | very faint phenolic |
| Odor Type | bland |
| Биологические источники | synthetic |
| Растворимость в воде | <0.1 g/100 mL at 17 ºC |
| Мерк | 14,1584 |
| Номер JECFA | 870 |
| БРН | 1103741 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, strong alkalies. |
| ИнЧИКей | QFOHBWFCKVYLES-UHFFFAOYSA-N |
| LogP | 3.57 |
| Вещества, добавляемые в пищу (ранее EAFUS) | BUTYL P-HYDROXYBENZOATE |
| FDA 21 CFR | 172.515 |
| Справочник по базе данных CAS | 94-26-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 5-7 |
| Словарь онкологических терминов NCI | SPF |
| FDA UNII | 3QPI1U3FV8 |
| Справочник по химии NIST | P-hydroxybenzoic acid, n-butyl ester(94-26-8) |
| Система регистрации веществ EPA | Butylparaben (94-26-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 22-24/25-37/39-26 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | DH1980000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29182900 | |||||||||
| Банк данных об опасных веществах | 94-26-8(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H318:При попадании в глаза вызывает необратимые последствия.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P332+P313:При возникновении раздражения кожи: обратиться за медицинской помощью.
P362+P364:Снять всю загрязненную одежду и выстирать ее перед повторным использованием.
Бутилпарабен химические свойства, назначение, производство
Описание
Butylparaben is an antimicrobial agent used in pharmaceutical suspensions. It is act by inhibiting DNA, RNA, and enzymes (eg, ATPase and phosphotransferase) synthesis. Butylparaben may be used alone or with other parabens, chiefly methylparaben and/or propylparaben, in medications. It is common in many liquid and solid (gel cap) OTC products such as Tylenol, Drixoral, Maalox, and Mylanta. Unfortunately, butylparaben concentrations were seldom identified for OTC or prescription products. No attempt was made to identify butylparaben-containing dietary supplements.Химические свойства
Butylparaben occurs as colorless crystals or a white, crystalline, odorless or almost odorless, tasteless powder.Подготовка
Butyl paraben is prepared by esterifying p-hydroxybenzoic acid with butyl alcohol in the presence of an acid catalyst, such as sulfuric acid, and an excess of the specific alcohol.Методы производства
Butylparaben is prepared by esterification of p-hydroxybenzoic acid with n-butanol.Общее описание
Odorless white crystals or crystalline powder. Tasteless, but numbs the tongue. Aqueous solutions slightly acidic to litmus.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Butylparaben is incompatible with strong oxidizing agents and strong caustics.Пожароопасность
Flash point data for Butylparaben are not available; however, Butylparaben is probably combustible.Фармацевтические приложения
Butylparaben is widely used as an antimicrobial preservative in cosmetics and pharmaceutical formulations.It may be used either alone or in combination with other paraben esters or with other antimicrobial agents. In cosmetics, it is the fourth most frequently used preservative.
As a group, the parabens are effective over a wide pH range and have a broad spectrum of antimicrobial activity, although they are most effective against yeasts and molds.
Owing to the poor solubility of the parabens, paraben salts, particularly the sodium salt, are frequently used in formulations. However, this may raise the pH of poorly buffered formulations. See Methylparaben for further information.
Контактные аллергены
This substance is one of the parabens family. Parabens are esters formed by p-hydroxybenzoic acid and an alcohol. They are largely used as biocides in cosmetics and toiletries, medicaments, or food. They have synergistic power with other biocides. Parabens can induce allergic contact dermatitis, mainly in chronic dermatitis and wounded skin.Безопасность
Butylparaben and other parabens are widely used as antimicrobial preservatives in cosmetics and oral and topical pharmaceutical formulations.Systemically, no adverse reactions to parabens have been reported, although they have been associated with hypersensitivity reactions generally appearing as contact dematitis. Immediate reactions with urticaria and bronchospasm have occurred rarely. See Methylparaben for further information.
LD50 (mouse, IP): 0.23 g/kg
LD50 (mouse, oral): 13.2 g/kg
хранилище
Aqueous butylparaben solutions at pH 3–6 can be sterilized by autoclaving, without decomposition. At pH 3–6, aqueous solutions are stable (less than 10% decomposition) for up to about 4 years at room temperature, while solutions at pH 8 or above are subject to rapid hydrolysis (10% or more after about 60 days at room temperature).Несовместимости
The antimicrobial activity of butylparaben is considerably reduced in the presence of nonionic surfactants as a result of micellization. Absorption of butylparaben by plastics has not been reported but appears probable given the behavior of other parabens. Some pigments, e.g. ultramarine blue and yellow iron oxide, absorb butylparaben and thus reduce its preservative properties.Butylparaben is discolored in the presence of iron and is subject to hydrolysis by weak alkalis and strong acids.
Регуляторный статус
Butylparaben is regulated by the U.S. Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA) and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). In 1998 its pesticide registration status was listed as "cancelled" (U.S. EPA, 2003).Included in the FDA Inactive Ingredients Database (injections; oral capsules, solutions, suspensions, syrups and tablets; rectal, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Бутилпарабен запасные части и сырье
Бутилпарабен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 510-85010237 | CHINA | 41 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 |