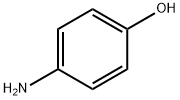
4-аминофенол
- английское имя4-Aminophenol
- CAS №123-30-8
- CBNumberCB5852965
- ФормулаC6H7NO
- мольный вес109.13
- EINECS204-616-2
- номер MDLMFCD00007869
- файл Mol123-30-8.mol
| Температура плавления | 188 °C |
| Температура кипения | 284 °C |
| плотность | 1.29 |
| давление пара | 0.4 hPa (110 °C) |
| показатель преломления | 1.5444 (estimate) |
| Fp | 189 °C |
| температура хранения | 2-8°C |
| растворимость | water: slightly soluble |
| форма | Crystalline Powder |
| пка | 5.48, 10.30(at 25℃) |
| цвет | White to cream |
| Растворимость в воде | 1.5 g/100 mL (20 ºC) |
| Чувствительный | Air & Light Sensitive |
| Разложение | 284 °C |
| Мерк | 14,462 |
| БРН | 385836 |
| Стабильность | Stable, though may discolour in air. Incompatible with acids, chloroformates, strong oxidizing agents. |
| ИнЧИКей | PLIKAWJENQZMHA-UHFFFAOYSA-N |
| LogP | -0.09-0.04 at 25℃ |
| Справочник по базе данных CAS | 123-30-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4-6 |
| FDA UNII | R7P8FRP05V |
| Справочник по химии NIST | Phenol, 4-amino-(123-30-8) |
| Система регистрации веществ EPA | p-Aminophenol (123-30-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,N | |||||||||
| Заявления о рисках | 20/22-50/53-68-40-R68-R50/53-R20/22 | |||||||||
| Заявления о безопасности | 28-36/37-60-61-28A-S61-S60-S36/37-S28A | |||||||||
| РИДАДР | UN 2512 6.1/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | SJ5075000 | |||||||||
| F | 8 | |||||||||
| Температура самовоспламенения | >250 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28402090 | |||||||||
| кода HS | 29222900 | |||||||||
| Банк данных об опасных веществах | 123-30-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 375 mg/kg LD50 dermal Rabbit > 10000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H302+H332:Вредно при проглатывании или при вдыхании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
4-аминофенол химические свойства, назначение, производство
Описание
4-Aminophenol, also known as 4-hydroxyaniline, is an organic building block. Its quantification in water samples upto the detection limit of 8×10-10mol l-1 has been proposed by employing single-wall carbon nanotubes (SWNT)-nafion film coated glassy carbon electrodes. It is present as the main contaminant in pharmaceutical formulations of paracetamol. High-performance liquid chromatographic (HPLC) method with amperometric detection has been reported for its determination in various analgesic formulations. It has been reported to be formed from the reduction of 4-nitrophenol (Nip) under metal-free conditions catalyzed by N-doped graphene (NG).Химические свойства
o-Aminophenol appears as colorless needles or as white crystalline substance turning tan to brown on exposure to air. slightly soluble in water and ethanol, insoluble in benzene and chloroform, and quickly turns brown when dissolved in lye.Использование
4-Aminophenol is suitable for use in the synthesis of 2,2-bis(4-aminophenoxy) benzonitrile [4-APBN], a monomer required for the preparation of series of polyamides and poly(amide-imide)s. It may be used as derivatization reagent to improve the ionization of aliphatic and aromatic aldehydes by paper spray ionization mass spectrometry.Подготовка
Conventionally 4-aminophenol was manufactured using iron-acid reduction of p-nitrobenzene. Reduction using iron-acid is a multi-step process. The modern method is the catalytic dehydrogenation of nitrobenzene to 4-aminophenol using a noble metal catalyst in the presence of an acidic medium. This method also produces aniline as a side-product. The advantage of a reduction using a noble metal catalyst is that it involves a single step reaction, an environment friendly and more efficient process, as there is no evolution of an environmentally harmful gas. Moreover, the side-product aniline is also a valuable chemical.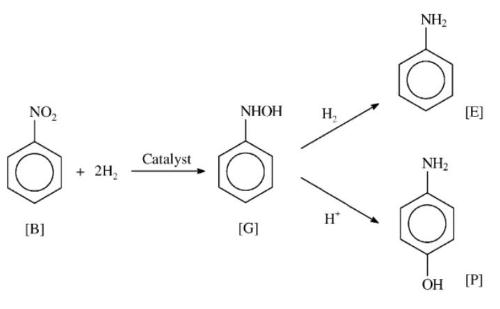
synthesis of 4-aminophenol
прикладной
The main and the most significant use of 4-Aminophenol is for the manufacturing of Paracetamol, an analgesic and antipyretic drug. In addition to paracetamol, it is a key element in the synthesis of pharmaceutical ingredients and important industrial chemicals like Acebutolol, Ambroxol, Sorafenib and so on.4-Aminophenol is used as a dye for textiles, hair, furs and feathers, and is also used as a developing agent in photography for creating black and white images. It acts as a corrosion inhibitor in paints and as anti-corrosive lubricating agent in 2-cycle engines. It is also used as a wood stain, giving rose-like colour to timber. p-Aminophenol is one of key ingredients for synthesis of rubber antioxidants. Moreover, it is often used as a reagent for analysing metals like Copper, Magnesium, Vanadium and Gold, compounds like Nitrites and Cyanates, and antioxidants.
Определение
ChEBI: 4-aminophenol is an amino phenol (one of the three possible isomers) which has the single amino substituent located para to the phenolic -OH group. It has a role as a metabolite and an allergen.Общее описание
P-aminophenol appears as white or reddish-yellow crystals or light brown powder. Turns violet when exposed to light. (NTP, 1992)Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Heat (decomposition forming HCN, nitrous vapors, CO); water (CO2); reacts violently with acids, bases, alcohols and amines causing fire and explosion hazards [Handling Chemicals Safely 1980 p. 647].Угроза здоровью
4-Aminophenol is of moderately low toxicity but has caused dermal sensitization and kidney injury; the potential for producing methemoglobin is of relatively minor importance.The oral LD50 in rats was 671 mg/kg.1 Effects included central nervous system depression. A solution of 2.5% applied to abraded skin of rabbits was a mild irritant.1 p- Aminophenol caused dermal sensitization in guinea pigs, and skin sensitization has been reported in humans.2,3 The dermal LD50 in rabbits was greater than 8 g/kg, which strongly suggests that absorption through the skin is minimal.4 Single nonlethal acute doses in rats produced proximal renal tubular necrosis of the pars recta.
Пожароопасность
Flash point data are not available for 4-Aminophenol. 4-Aminophenol is probably combustible.Контактные аллергены
This hair dye is frequently implicated in contact dermatitis in hairdressers, customers, or people sensitized to para-phenylenediamine, by the way of “blackhenna” temporary tattoos.Профиль безопасности
Poison by ingestion, subcutaneous, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. An allergen and skin and eye irritant. Mutation data reported. Can cause contact dermatitis, bronchial asthma, and methemoglobinemia with cyanosis. When heated to decomposition it emits toxic fumes of NOx,.Возможный контакт
Workers may be exposed to oAminophenol during its use as a chemical intermediate; in the manufacture of azo and sulfur dyes; and in the photographic industry. There is potential for consumer exposure to o-Aminophenol because of its use in dyeing hair, fur, and leather. The compound is a constituent of 75 registered cosmetic products suggesting the potential for widespread consumer exposure. p-Aminophenol is used mainly as a dye, dye intermediate and as a photographic developer; and in small quantities in analgesic drug preparation. Consumer exposure to p-aminophenol may occur from use as a hairdye or as a component in cosmetic preparations. m-Aminophenol is used mainly as a dye intermediate.Растворимость в органика
Very soluble in dimethylsulfoxide Soluble in acetonitrile, ethyl acetate, and acetone Slightly soluble in toluene, diethyl ether, and ethanol Negligible solubility in benzene and chloroformПеревозки
UN2512 Aminophenols (o-; m-; p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materialsМетоды очистки
Crystallise it from EtOH, then water, excluding oxygen. It sublimes at 110o/0.3mm. It has been purified by chromatography on alumina with a 1:4 (v/v) mixture of absolute EtOH/*benzene as eluent. [Beilstein 13 IV 1014.]Несовместимости
These phenol/cresol materials can react with oxidizers; reaction may be violent. Incompatible with strong reducing substances such as alkali metals, hydrides, nitrides, and sulfides. Flammable gas (H2) may be generated, and the heat of the reaction may cause the gas to ignite and explode. Heat may be generated by the acidbase reaction with bases; such heating may initiate polymerization of the organic compound. Reacts with boranes, alkalies, aliphatic amines, amides, nitric acid, sulfuric acid. Phenols are sulfonated very readily (e.g., by concentrated sulfuric acid at room temperature). These reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid and can explode when heated. Many phenols form metal salts that may be detonated by mild shock.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.4-аминофенол запасные части и сырье
сырьё
1of3
запасной предмет
- IMMEDIAL BRILLIANT GREEN GB
- DIAMINE GREEN B
- Sulphur Red 6
- 2-Hydroxy-N-(4-hydroxyphenyl)-benzamide
- THIFLUZAMIDE
- N-(1,3-ДИМЕТИЛБУТИЛ)-N'-ФЕНИЛ-p-ФЕНИЛЕНДИАМИН
- Sulphur Black 6
- Роксарсон
- 4-метиламинофенол сульфа
- Sulfur Red Brown B3R
1of5
4-аминофенол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0523-86392777 +86-19825580222 |
China | 23 | 58 | ||
| +8618922120635 | China | 998 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |